![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)
BRIEF ANSWERS TO SELECTED PROBLEMS APPENDIX G
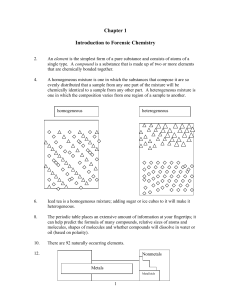
... 1.2 Gas molecules fill the entire container; the volume of a gas is the volume of the container. Solids and liquids have a definite volume. The volume of the container does not affect the volume of a solid or liquid. (a) gas (b) liquid (c) liquid 1.4 Physical property: a characteristic shown by a su ...
... 1.2 Gas molecules fill the entire container; the volume of a gas is the volume of the container. Solids and liquids have a definite volume. The volume of the container does not affect the volume of a solid or liquid. (a) gas (b) liquid (c) liquid 1.4 Physical property: a characteristic shown by a su ...
chapter 18 - HCC Learning Web
... Magnesium is an alkaline earth metal; Mg will oxidize to Mg2+. The oxidation state of hydrogen in HCl is +1. To be reduced, the oxidation state of H must decrease. The obvious choice for the hydrogen product is H2(g), where hydrogen has a zero oxidation state. The balanced reaction is Mg(s) + 2HCl(a ...
... Magnesium is an alkaline earth metal; Mg will oxidize to Mg2+. The oxidation state of hydrogen in HCl is +1. To be reduced, the oxidation state of H must decrease. The obvious choice for the hydrogen product is H2(g), where hydrogen has a zero oxidation state. The balanced reaction is Mg(s) + 2HCl(a ...
44. Find рН of formic acid solution with mass percent ω=5
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
CHAPTER 9 Stoichiometry - Modern Chemistry Textbook
... reaction-stoichiometry calculations described in this chapter are theoretical. They tell us the amounts of reactants and products for a given chemical reaction under ideal conditions, in which all reactants are completely converted into products. However, ideal conditions are rarely met in the labor ...
... reaction-stoichiometry calculations described in this chapter are theoretical. They tell us the amounts of reactants and products for a given chemical reaction under ideal conditions, in which all reactants are completely converted into products. However, ideal conditions are rarely met in the labor ...
Chem Soc Rev
... acids are produced via selective oxidation catalysis. Among the selective oxidation processes in the current chemical industry, the selective oxidation of propene to acrolein, the ammoxidation of propene to acrylonitrile, the selective oxidation of butane to maleic anhydride, the epoxidation of ethy ...
... acids are produced via selective oxidation catalysis. Among the selective oxidation processes in the current chemical industry, the selective oxidation of propene to acrolein, the ammoxidation of propene to acrylonitrile, the selective oxidation of butane to maleic anhydride, the epoxidation of ethy ...
chapter 5 gases
... Strategy: Compression is work done on the gas, so what is the sign for w? Heat is released by the gas to the surroundings. Is this an endothermic or exothermic process? What is the sign for q? Solution: To calculate the energy change of the gas (U), we need Equation (6.1) of the text. Work of compr ...
... Strategy: Compression is work done on the gas, so what is the sign for w? Heat is released by the gas to the surroundings. Is this an endothermic or exothermic process? What is the sign for q? Solution: To calculate the energy change of the gas (U), we need Equation (6.1) of the text. Work of compr ...
master ap chemistry - NelnetSolutions.com
... For more information, contact Peterson’s, 2000 Lenox Drive, Lawrenceville, NJ 08648; 800-338-3282; or find us on the World Wide Web at: www.petersons.com/about. © 2007 Peterson’s, a Nelnet company Previous edition © 2005 Editor: Wallie Walker Hammond; Production Editor: Mark D. Snider; Composition M ...
... For more information, contact Peterson’s, 2000 Lenox Drive, Lawrenceville, NJ 08648; 800-338-3282; or find us on the World Wide Web at: www.petersons.com/about. © 2007 Peterson’s, a Nelnet company Previous edition © 2005 Editor: Wallie Walker Hammond; Production Editor: Mark D. Snider; Composition M ...
4134gdisk doc..4134gdisk chapter .. Page501
... combination of stopped flow technology and EXAFS has allowed the investigation of the structure of short-lived intermediates in some redox processes.76 Evidence has been produced that under conditions where V(V) is present as both VO2+ and decavanadite in the reaction with Fe(II), the latter are als ...
... combination of stopped flow technology and EXAFS has allowed the investigation of the structure of short-lived intermediates in some redox processes.76 Evidence has been produced that under conditions where V(V) is present as both VO2+ and decavanadite in the reaction with Fe(II), the latter are als ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.