REVIEWS Environmental remediation by photocatalysis R. Vinu AND Giridhar Madras
... species”. When the reactions are carried out in non-aqueous (organic) medium, the surface bound hydroxyl species present in the semiconductor plays a major role (reaction (2)), and the contribution of reactions (8)–(14) for the overall oxidation of the substrate is negligible. Once the active specie ...
... species”. When the reactions are carried out in non-aqueous (organic) medium, the surface bound hydroxyl species present in the semiconductor plays a major role (reaction (2)), and the contribution of reactions (8)–(14) for the overall oxidation of the substrate is negligible. Once the active specie ...
b - Gordon State College
... a) If required > available, then B is the limiting reagent and A is the excess reagent. b) If required < available, then B is the excess reagent and A is the limiting reagent. 5) Use the amount of the limiting reagent and the stoichiometry to calculate the amount of any product and the amount of the ...
... a) If required > available, then B is the limiting reagent and A is the excess reagent. b) If required < available, then B is the excess reagent and A is the limiting reagent. 5) Use the amount of the limiting reagent and the stoichiometry to calculate the amount of any product and the amount of the ...
Corrosion of Ceramic and Composite Materials, Second Edition
... dissolution of various raw materials in molten glass in the manufacture of glass products. The proper selection of materials and good design practices can greatly reduce the cost caused by corrosion. To make the proper selection, engineers must be knowledgeable in the fields of thermodynamics, physi ...
... dissolution of various raw materials in molten glass in the manufacture of glass products. The proper selection of materials and good design practices can greatly reduce the cost caused by corrosion. To make the proper selection, engineers must be knowledgeable in the fields of thermodynamics, physi ...
The Impact of Ligand Design on the Coordination Chemistry and
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... Ans. A substance which contains only one kind of atom or molecule is called a pure substance . Q4- Define average atomic mass. (L-1) Ans. Average atomic mass is the average of atomic mass of all the isotopes of an element. Q5- What is one a.m.u. or one ‘u ,? (L-1) Ans:- One a.m.u. or u is equal to 1 ...
... Ans. A substance which contains only one kind of atom or molecule is called a pure substance . Q4- Define average atomic mass. (L-1) Ans. Average atomic mass is the average of atomic mass of all the isotopes of an element. Q5- What is one a.m.u. or one ‘u ,? (L-1) Ans:- One a.m.u. or u is equal to 1 ...
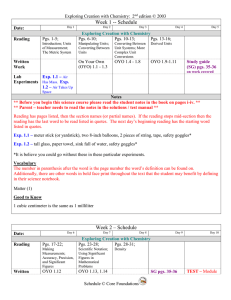
Week 1 -- Schedule
... ** Before you begin this science course please read the student notes in the book on pages i-iv. ** ** Parent – teacher needs to read the notes in the solutions / test manual ** Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has ...
... ** Before you begin this science course please read the student notes in the book on pages i-iv. ** ** Parent – teacher needs to read the notes in the solutions / test manual ** Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has ...
questions based on high order thinking skill
... Ans. Ethyl alcohol and water (95.4% ethyl alcohol and 4.6% water) form constant boiling mixture (azeotrope) boiling at 351.1 °K. Hence, further water cannot be separated completely from ethyl alcohol by fractional distillation. Q. 5. Why a person suffering from high blood pressure is advised to take ...
... Ans. Ethyl alcohol and water (95.4% ethyl alcohol and 4.6% water) form constant boiling mixture (azeotrope) boiling at 351.1 °K. Hence, further water cannot be separated completely from ethyl alcohol by fractional distillation. Q. 5. Why a person suffering from high blood pressure is advised to take ...
questions based on high order thinking skill - Entrance
... Ans. Ethyl alcohol and water (95.4% ethyl alcohol and 4.6% water) form constant boiling mixture (azeotrope) boiling at 351.1 °K. Hence, further water cannot be separated completely from ethyl alcohol by fractional distillation. Q. 5. Why a person suffering from high blood pressure is advised to take ...
... Ans. Ethyl alcohol and water (95.4% ethyl alcohol and 4.6% water) form constant boiling mixture (azeotrope) boiling at 351.1 °K. Hence, further water cannot be separated completely from ethyl alcohol by fractional distillation. Q. 5. Why a person suffering from high blood pressure is advised to take ...
Question Bank (Class XI - Chemistry)
... Ans. A substance which contains only one kind of atom or molecule is called a pure substance . Q4- Define average atomic mass. (L-1) Ans. Average atomic mass is the average of atomic mass of all the isotopes of an element. Q5- What is one a.m.u. or one ‘u ,? (L-1) Ans:- One a.m.u. or u is equal to 1 ...
... Ans. A substance which contains only one kind of atom or molecule is called a pure substance . Q4- Define average atomic mass. (L-1) Ans. Average atomic mass is the average of atomic mass of all the isotopes of an element. Q5- What is one a.m.u. or one ‘u ,? (L-1) Ans:- One a.m.u. or u is equal to 1 ...
Soln Chem 2008Nov(9746)
... from Cl2 to I2 due to stronger intermolecular van der Waals' forces as the number of electrons increases from Cl2 to I2. From Cl to I, electron affinity becomes less negative due to the increase in atomic size and hence, weaker attraction for the additional electron. (ans) © Step-by-Step ...
... from Cl2 to I2 due to stronger intermolecular van der Waals' forces as the number of electrons increases from Cl2 to I2. From Cl to I, electron affinity becomes less negative due to the increase in atomic size and hence, weaker attraction for the additional electron. (ans) © Step-by-Step ...
Section 1.3 - The Student Room
... a Standard enthalpy change of combustion is the enthalpy change when 1 mole of the compound is burnt completely in oxygen, under standard conditions (ie the compound and the products in their most stable states at 1 atmosphere pressure and at a stated temperature, often 298 K). b Standard enthalpy c ...
... a Standard enthalpy change of combustion is the enthalpy change when 1 mole of the compound is burnt completely in oxygen, under standard conditions (ie the compound and the products in their most stable states at 1 atmosphere pressure and at a stated temperature, often 298 K). b Standard enthalpy c ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.