Properties of Systems in Equilibrium - Le
... tube. To confirm that the solid is present, let the test tube sit on the bench for about 3 minutes, allowing all solid to settle to the bottom where it is easier to see. On your data sheet record the total volume of 0.3 M HCl needed to produce the solid. 6. Put the test tube containing the solid int ...
... tube. To confirm that the solid is present, let the test tube sit on the bench for about 3 minutes, allowing all solid to settle to the bottom where it is easier to see. On your data sheet record the total volume of 0.3 M HCl needed to produce the solid. 6. Put the test tube containing the solid int ...
1 - Academics
... 11. A covalent bond is best described as: a) The complete transfer of a pair of e- between two atoms; b) The complete transfer of a single e- between two atoms; c) The sharing of a single e- between two atoms; d) When an electron falls into the nucleus of another atom. e) The sharing of a pair of e- ...
... 11. A covalent bond is best described as: a) The complete transfer of a pair of e- between two atoms; b) The complete transfer of a single e- between two atoms; c) The sharing of a single e- between two atoms; d) When an electron falls into the nucleus of another atom. e) The sharing of a pair of e- ...
Glossary: Chemical bonds
... equilibrium will be shifted in the direction which weakens the external influence. The increase in temperature shifts the equilibrium towards an endothermic reaction (the system absorbs heat and increases its internal energy, H>0), and decrease in temperature shifts the equilibrium towards an exoth ...
... equilibrium will be shifted in the direction which weakens the external influence. The increase in temperature shifts the equilibrium towards an endothermic reaction (the system absorbs heat and increases its internal energy, H>0), and decrease in temperature shifts the equilibrium towards an exoth ...
Example 1-2
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
Chemical equilibrium and the kinetic theory of gases
... Equilibrium processes have a central importance to industrial chemistry. Although reactions are rarely allowed to reach equilibrium, knowledge of the factors that influence the position of equilibrium is critical for a chemical engineer. In this unit you will become familiar with various quantitativ ...
... Equilibrium processes have a central importance to industrial chemistry. Although reactions are rarely allowed to reach equilibrium, knowledge of the factors that influence the position of equilibrium is critical for a chemical engineer. In this unit you will become familiar with various quantitativ ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) • The only things left in the equation are those things that change (i.e., react) durin ...
... Net Ionic Equation • To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) • The only things left in the equation are those things that change (i.e., react) durin ...
CHEMICAL REACTIONS AND CHEMICAL EQUATIONS
... industry , there, it is used to do cost and analysis for manufacturing chemicals. In fact, manufacturing processes are financed according to the cost of reactants and the values of products are determined by stoichiometric calculations. STOICHIOMETRY : The relationship of quantities ( mass of substa ...
... industry , there, it is used to do cost and analysis for manufacturing chemicals. In fact, manufacturing processes are financed according to the cost of reactants and the values of products are determined by stoichiometric calculations. STOICHIOMETRY : The relationship of quantities ( mass of substa ...
File
... • The following reaction shows table salt production. How many moles of sodium chloride are produced from 0.02 moles of chlorine? ...
... • The following reaction shows table salt production. How many moles of sodium chloride are produced from 0.02 moles of chlorine? ...
111 Exam III OUTLINE TRO 1-3-11
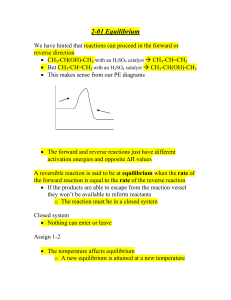
... 1. The forward reaction (⇀ ) and reverse (↽ ) reactions are occurring simultaneously. 2. The rate for the forward reaction is equal to the rate of the reverse reaction and a dynamic equilibrium is achieved. 3. The ratio of the concentrations of the products to reactants is constant. B. THE EQUILIBRI ...
... 1. The forward reaction (⇀ ) and reverse (↽ ) reactions are occurring simultaneously. 2. The rate for the forward reaction is equal to the rate of the reverse reaction and a dynamic equilibrium is achieved. 3. The ratio of the concentrations of the products to reactants is constant. B. THE EQUILIBRI ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.