Chapter 7 - Chemical Quantities
... b) How many grams of H2 are needed to react with 2.80 g of N2? ...
... b) How many grams of H2 are needed to react with 2.80 g of N2? ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... c) isotopic abundance & relative atomic mass d) empirical & molecular formula e) law of definite proportions or constant composition f) quantitative relationships in a balanced equation g) limiting reagent h) actual yield, theoretical yield, percentage yield 2. A sample of glucose (C6H12O6) has a ma ...
... c) isotopic abundance & relative atomic mass d) empirical & molecular formula e) law of definite proportions or constant composition f) quantitative relationships in a balanced equation g) limiting reagent h) actual yield, theoretical yield, percentage yield 2. A sample of glucose (C6H12O6) has a ma ...
honors final key
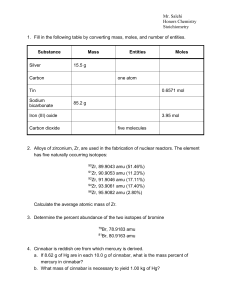
... 17. Convert the following a. 4.58 mol to particles of Al = 2.76x1024 b. 4.58 mol to grams of Al = 124g c. 901.49 g to particles of Carbon dioxide = 1.23x 1025 d. 901.49 g to L of carbon dioxide = 458.9 18. Define molar mass. The mass of a mole of a substance, equal to the atomic mass in grams 19. Gi ...
... 17. Convert the following a. 4.58 mol to particles of Al = 2.76x1024 b. 4.58 mol to grams of Al = 124g c. 901.49 g to particles of Carbon dioxide = 1.23x 1025 d. 901.49 g to L of carbon dioxide = 458.9 18. Define molar mass. The mass of a mole of a substance, equal to the atomic mass in grams 19. Gi ...
Chemical Equations and Reactions
... An amount of an element or compound in moles can be converted to a mass in grams by multiplying by the appropriate molar mass. • example: ...
... An amount of an element or compound in moles can be converted to a mass in grams by multiplying by the appropriate molar mass. • example: ...
honors chem 6 day review packet
... the element that has 2 electrons in the p sublevel in its second main energy level 4s24p5 Be able to locate s, p, d, and f blocks on the periodic table The ___________ ____________ _____________ is the same as the period number. There are ________ main energy levels. The ____________ ___________ ___ ...
... the element that has 2 electrons in the p sublevel in its second main energy level 4s24p5 Be able to locate s, p, d, and f blocks on the periodic table The ___________ ____________ _____________ is the same as the period number. There are ________ main energy levels. The ____________ ___________ ___ ...
50 Frequently Forgotten Facts
... a) Quantitative analysis determines that a compound has an empirical formula of CH and a molecular mass of 26 grams/mole. Determine the molecular formula of this compound, showing all work: ...
... a) Quantitative analysis determines that a compound has an empirical formula of CH and a molecular mass of 26 grams/mole. Determine the molecular formula of this compound, showing all work: ...
sample paper chemistry clas xi set 3
... Due to small size if Na and K, less energy required for the excitation of e -, whereas in Mg atom, due to small size, large amount ofenergy is required ...
... Due to small size if Na and K, less energy required for the excitation of e -, whereas in Mg atom, due to small size, large amount ofenergy is required ...
FREQUENTLY FORGOTTEN FACTS
... a) Quantitative analysis determines that a compound has an empirical formula of CH and a molecular mass of 26 grams/mole. Determine the molecular formula of this compound, showing all work: ...
... a) Quantitative analysis determines that a compound has an empirical formula of CH and a molecular mass of 26 grams/mole. Determine the molecular formula of this compound, showing all work: ...
Stoichiometry - Cloudfront.net
... 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 mg H2O on combustion. The compound contains 4.680% N by mass. Is the white solid cocaine? 8. An unknown compound (molar mass = 176 g/mol) contains 68.2 mass % C, 6.86 mass ...
... 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 mg H2O on combustion. The compound contains 4.680% N by mass. Is the white solid cocaine? 8. An unknown compound (molar mass = 176 g/mol) contains 68.2 mass % C, 6.86 mass ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.