C2 Revision Quick Questions FT
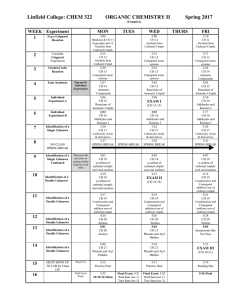
... The relative atomic mass of an element (Ar) compares the mass of atoms of the element, has the same value as the mass number. The relative formula mass (Mr) of a compound is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. The relative formula mass of a substan ...
... The relative atomic mass of an element (Ar) compares the mass of atoms of the element, has the same value as the mass number. The relative formula mass (Mr) of a compound is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. The relative formula mass of a substan ...
DEPARTMENT OF CHEMISTRY
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
Final Exam Study Guide Page 1 Quiz
... a. The number of atoms in a mole of an element b. The number of molecules in a mole of a compound c. A and B d. None of the above Use the following equation to answer numbers 9, 10, and 11: Fe + 2H2SO4 → Fe(SO4)2 +2 H2 9. If 2.31g iron reacted with 8.83g sulfuric acid, what is the limiting reactant? ...
... a. The number of atoms in a mole of an element b. The number of molecules in a mole of a compound c. A and B d. None of the above Use the following equation to answer numbers 9, 10, and 11: Fe + 2H2SO4 → Fe(SO4)2 +2 H2 9. If 2.31g iron reacted with 8.83g sulfuric acid, what is the limiting reactant? ...
Chapter 3 - Bruder Chemistry
... The quantitative nature of chemical formulas and reactions is called stoichiometry. Lavoisier observed that mass is conserved in a chemical reaction. • This observation is known as the law of conservation of mass. Chemical equations give a description of a chemical reaction. There are two parts to a ...
... The quantitative nature of chemical formulas and reactions is called stoichiometry. Lavoisier observed that mass is conserved in a chemical reaction. • This observation is known as the law of conservation of mass. Chemical equations give a description of a chemical reaction. There are two parts to a ...
Part II - American Chemical Society
... c. Calculate the rate constant for this reaction at 75 ˚C. d. The following two-step mechanism has been proposed for this reaction: Step 1 O3(g) → O2(g) + O(g) NO(g) + O(g) → NO2(g) Step 2 State and explain whether this mechanism is consistent with the observed rate law. ...
... c. Calculate the rate constant for this reaction at 75 ˚C. d. The following two-step mechanism has been proposed for this reaction: Step 1 O3(g) → O2(g) + O(g) NO(g) + O(g) → NO2(g) Step 2 State and explain whether this mechanism is consistent with the observed rate law. ...
chemistry 11 exam review
... 12. To what temperature must 15 L of oxygen gas at 0.0C be heated at 1.00 atm pressure in order to occupy a volume of 23 L, assuming that the pressure increases by 5.0 mm Hg? (421 K = 148C) 13. Determine the volume of a balloon at SATP assuming that it occupies a volume of 20.0 L at a temperature ...
... 12. To what temperature must 15 L of oxygen gas at 0.0C be heated at 1.00 atm pressure in order to occupy a volume of 23 L, assuming that the pressure increases by 5.0 mm Hg? (421 K = 148C) 13. Determine the volume of a balloon at SATP assuming that it occupies a volume of 20.0 L at a temperature ...
I - Holland Public Schools
... In this case, 2 C2H2’s and 5 O2’s would need to collide in the same place at the same time VERY UNLIKELY * OK, so how does this work then? The chemical reaction is divided into a series of steps, each of which produces an intermediate, a product that is used as a reactant in a later step. Each step ...
... In this case, 2 C2H2’s and 5 O2’s would need to collide in the same place at the same time VERY UNLIKELY * OK, so how does this work then? The chemical reaction is divided into a series of steps, each of which produces an intermediate, a product that is used as a reactant in a later step. Each step ...
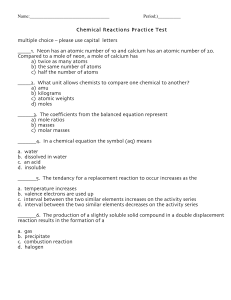
Chap. 4 - Chemical Reactions
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
Aqueous Solutions
... balance elements except H and O balance O atoms by adding H2O balance H atoms by adding H+ add 1 OH- to both sides for every H+ added combine H+ and OH- on same side to make H2O cancel the same # of H2O from each side balance charge by adding e- to side with greater overall ...
... balance elements except H and O balance O atoms by adding H2O balance H atoms by adding H+ add 1 OH- to both sides for every H+ added combine H+ and OH- on same side to make H2O cancel the same # of H2O from each side balance charge by adding e- to side with greater overall ...
$doc.title
... side of the arrow (and also the number of oxygen atoms on the left side of the arrow), equals the number of hydrogen atoms (and likewise the number of oxygen atoms), on the right side of the arrow. Atoms are the smallest units of matter that retain chemical properties. Atoms are not visible under n ...
... side of the arrow (and also the number of oxygen atoms on the left side of the arrow), equals the number of hydrogen atoms (and likewise the number of oxygen atoms), on the right side of the arrow. Atoms are the smallest units of matter that retain chemical properties. Atoms are not visible under n ...
AP Chemistry Syllabus
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as freshman, second year work in the chemistry sequence at their institution or to register in courses in ...
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as freshman, second year work in the chemistry sequence at their institution or to register in courses in ...
Introduction to Chemistry and the Metric System
... shared pair of electrons, unshared pair, single bond, double bond, triple bond VSEPR Theory, hybrid orbitals, shapes of molecules, sigma bonds, pi bonds, polarity Intermolecular Forces (in order from weakest to strongest): London Dispersion Forces, dipole-dipole interactions, H-bonding, ionic ...
... shared pair of electrons, unshared pair, single bond, double bond, triple bond VSEPR Theory, hybrid orbitals, shapes of molecules, sigma bonds, pi bonds, polarity Intermolecular Forces (in order from weakest to strongest): London Dispersion Forces, dipole-dipole interactions, H-bonding, ionic ...
Measuring and Calculating
... equal sharing of the electron pair and thus no partial charge development, (often C-H) example: nonpolar covalent bonds are found in methane, CH 4, and nitrogen, N2. Polar covalent bond – larger electronegativity difference, results in an unequal sharing of the electron pair and thus partial charg ...
... equal sharing of the electron pair and thus no partial charge development, (often C-H) example: nonpolar covalent bonds are found in methane, CH 4, and nitrogen, N2. Polar covalent bond – larger electronegativity difference, results in an unequal sharing of the electron pair and thus partial charg ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.