Conductivities and transmission coefficients of ultra-thin disordered metallic films B. J.
... research, and on the other hand their potential applications in nanoscale technology bring about the rapid development of experimental techniques strongly supported by theoretical methods of analysing their electronic properties. One of the simplest examples of such a system is a thin film. In these ...
... research, and on the other hand their potential applications in nanoscale technology bring about the rapid development of experimental techniques strongly supported by theoretical methods of analysing their electronic properties. One of the simplest examples of such a system is a thin film. In these ...
Chapter 4 - Tolland High School
... Energy States of Atoms/Electrons • Ground State- lowest energy level of an electron within an atom • Excited State- a higher energy level within an atom that an electron may exist in – Energy must be absorbed for an electron to go from ground to excited state – Energy is given off as visible light ...
... Energy States of Atoms/Electrons • Ground State- lowest energy level of an electron within an atom • Excited State- a higher energy level within an atom that an electron may exist in – Energy must be absorbed for an electron to go from ground to excited state – Energy is given off as visible light ...
CHAPTER 8 PERIODIC RELATIONSHIPS AMONG THE ELEMENTS
... Hence, when a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n−1)d orbitals if necessary. Since the metal ion has a +3 charge, three electrons have been removed. Since the 4s subshell is less stable than the 3d, two elec ...
... Hence, when a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n−1)d orbitals if necessary. Since the metal ion has a +3 charge, three electrons have been removed. Since the 4s subshell is less stable than the 3d, two elec ...
pptx
... DFT is a ground-state theory for electrons But many processes involve exciting electrons: • Transport of electrons, electron energy levels • Excited electrons ...
... DFT is a ground-state theory for electrons But many processes involve exciting electrons: • Transport of electrons, electron energy levels • Excited electrons ...
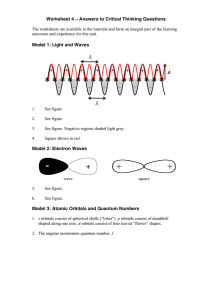
Answers to Critical Thinking Questions 4
... b. Pauli Exclusion Principle: no two electrons may have the same set of quantum numbers. No orbital can accommodate more than 2 electrons. c. Hund’s Rule: in a set of orbitals with the same energy, electrons go into different orbitals with aligned spins until each orbital in the set contains one ele ...
... b. Pauli Exclusion Principle: no two electrons may have the same set of quantum numbers. No orbital can accommodate more than 2 electrons. c. Hund’s Rule: in a set of orbitals with the same energy, electrons go into different orbitals with aligned spins until each orbital in the set contains one ele ...
final exam kérdések: 1.)There are n photons in a cavity composed of
... a.) The energy of photon is about E1 eV b.) The average number of photons are about N million photons/sec. c.) If the signal is the average number of photons, the coherent noise level is E1 pico watt, and the SNR is about A d.)The direction of the momentum vector is -Z e.)In case of a collision with ...
... a.) The energy of photon is about E1 eV b.) The average number of photons are about N million photons/sec. c.) If the signal is the average number of photons, the coherent noise level is E1 pico watt, and the SNR is about A d.)The direction of the momentum vector is -Z e.)In case of a collision with ...
Semester 1 Final Exam Study Guide
... 31. How many valence electrons do most atoms need to become as stable as possible? 32. What part of the atom is involved in compound formation? ...
... 31. How many valence electrons do most atoms need to become as stable as possible? 32. What part of the atom is involved in compound formation? ...
Chapter 6 Electronic Structure of Atoms
... The Principle Quantum Number gives us the overall size of the electrons probability density; distance from the nucleus. The Azmuthal Quantum Number (Angular-Momentum) describes the number of probability densities at any given main energy level; the number and shapes of electron sublevel energies ...
... The Principle Quantum Number gives us the overall size of the electrons probability density; distance from the nucleus. The Azmuthal Quantum Number (Angular-Momentum) describes the number of probability densities at any given main energy level; the number and shapes of electron sublevel energies ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... (II) If it is ionic, list the ions involved in the bonding (III) If it is covalent, draw the Lewis structure, and state if it has polar or nonpolar bonds. (a) MgCl2 (g) Na3N ...
... (II) If it is ionic, list the ions involved in the bonding (III) If it is covalent, draw the Lewis structure, and state if it has polar or nonpolar bonds. (a) MgCl2 (g) Na3N ...
NUCLEAR PHYSICS
... momenta are no longer constants of motion, but the sum of the two angular momenta usually still is. Angular momentum coupling in atoms is of importance in atomic spectroscopy. Angular momentum coupling of electron spins is of importance in quantum chemistry. Also in the nuclear shell model angular m ...
... momenta are no longer constants of motion, but the sum of the two angular momenta usually still is. Angular momentum coupling in atoms is of importance in atomic spectroscopy. Angular momentum coupling of electron spins is of importance in quantum chemistry. Also in the nuclear shell model angular m ...
Qubits based on electrons on helium The basic building block of a
... basis states, by ionizing the excited state electrons onto a multi-channel plate (MCP) detector. We propose to use an RF-SET as the readout system also for this type of qubit. This means that we will not lose the electron from the trap (which would be a real problem when we want to do error correcti ...
... basis states, by ionizing the excited state electrons onto a multi-channel plate (MCP) detector. We propose to use an RF-SET as the readout system also for this type of qubit. This means that we will not lose the electron from the trap (which would be a real problem when we want to do error correcti ...
Chapter 7, Quantum Nos.
... For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, ...
... For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for multi-electron atoms. But since these atoms have more than one electron, ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.