Test 4 Review
... Covalent Bonds. Covalent bonds are bonds formed by sharing electrons. The electrons of one atom are attracted to the protons of another, but neither atom pulls strongly enough to remove an electron from the other. Covalent bonds form when the electronegativity difference between the elements is less ...
... Covalent Bonds. Covalent bonds are bonds formed by sharing electrons. The electrons of one atom are attracted to the protons of another, but neither atom pulls strongly enough to remove an electron from the other. Covalent bonds form when the electronegativity difference between the elements is less ...
Orbitals
... Matter and Energy By 1900, physicists thought that the nature of energy and matter was well understood and distinct. Matter, a collection of particles, have mass and a defined position in space. Radiant energy, as waves, is massless and delocalized. It was also believed that particles of matter cou ...
... Matter and Energy By 1900, physicists thought that the nature of energy and matter was well understood and distinct. Matter, a collection of particles, have mass and a defined position in space. Radiant energy, as waves, is massless and delocalized. It was also believed that particles of matter cou ...
The Atom and Its Properties
... emitted from heated objects • Matter can only gain or lose energy in small specific amounts ...
... emitted from heated objects • Matter can only gain or lose energy in small specific amounts ...
الرقم الجامعي
... ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------ ...
... ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------ ...
Introduction to Quantum Mechanic
... of an electron exactly. Rather, it provides only a probability as to where the electron will be found. We shall illustrate the probability aspect in terms of the system of an electron confined to motion along a line of length L. Quantum mechanical probabilities are expressed in terms of a distributi ...
... of an electron exactly. Rather, it provides only a probability as to where the electron will be found. We shall illustrate the probability aspect in terms of the system of an electron confined to motion along a line of length L. Quantum mechanical probabilities are expressed in terms of a distributi ...
The Basics - I`m a faculty member, and I need web space. What
... and remove an electron from a gaseous atom • 1st ionization energy: the energy required to remove the first electron • 2nd ionization energy: the energy required to remove the second electron • 3rd ionization energy: the energy required removing the third electron • Trend: ionization energy increase ...
... and remove an electron from a gaseous atom • 1st ionization energy: the energy required to remove the first electron • 2nd ionization energy: the energy required to remove the second electron • 3rd ionization energy: the energy required removing the third electron • Trend: ionization energy increase ...
7B35.75 Plasma Tubes
... the electrode, which places the electrode at a much higher potential than the surrounding glass. There now exists a strong electric field within the glass dome. The electric field is strong enough to ionize some of the inert gas particles, leaving a neutral mixture of electrons and ions. This ionize ...
... the electrode, which places the electrode at a much higher potential than the surrounding glass. There now exists a strong electric field within the glass dome. The electric field is strong enough to ionize some of the inert gas particles, leaving a neutral mixture of electrons and ions. This ionize ...
Unit 1
... http://www.youtube.com/v/UyDnnD3fMaU&feature=PlayList&p=5DD6329BBE0DE93F&playnext_from=PL&playnext=1&index=1 ...
... http://www.youtube.com/v/UyDnnD3fMaU&feature=PlayList&p=5DD6329BBE0DE93F&playnext_from=PL&playnext=1&index=1 ...
Atomic Theory Notes Packet
... d block: Groups _____ through _____ filling (includes the ____________ elements) f block: _______________ and _______________ Series filling 2. Belated filling: when an ____ sublevel fills before a lower p sublevel. Example: 4s fills before the _____ 3. Valence Electrons: electrons in the __________ ...
... d block: Groups _____ through _____ filling (includes the ____________ elements) f block: _______________ and _______________ Series filling 2. Belated filling: when an ____ sublevel fills before a lower p sublevel. Example: 4s fills before the _____ 3. Valence Electrons: electrons in the __________ ...
Kondo effect of an antidot in the integer quantum Hall regime: a
... polarization is equivalent to increasing electron spin polarization. However, this result does not match those expected in the experiment [8] of large-size realistic antidots and the results of the capacitive interaction model [12]. In large-size antidots, it is naturally expected that the spin pola ...
... polarization is equivalent to increasing electron spin polarization. However, this result does not match those expected in the experiment [8] of large-size realistic antidots and the results of the capacitive interaction model [12]. In large-size antidots, it is naturally expected that the spin pola ...
Selective field ionization in Li and Rb: Theory and experiment
... Selective field ionization 共SFI兲 has been used as an experimental tool to measure the character and population of highly excited states of atoms and molecules. In SFI, the atom is subjected to an electric field that ramps to higher strengths over times that are very long compared to the classical pe ...
... Selective field ionization 共SFI兲 has been used as an experimental tool to measure the character and population of highly excited states of atoms and molecules. In SFI, the atom is subjected to an electric field that ramps to higher strengths over times that are very long compared to the classical pe ...
Schrodinger models of the atom
... Within an atom are major energy levels called shells. Each shell is assigned a number (called the principal quantum number), starting at the nucleus and increasing outwards (1, 2, 3...). The maximum number of electrons for any shell is given by the formula 2n2 where n stands for the shell number ...
... Within an atom are major energy levels called shells. Each shell is assigned a number (called the principal quantum number), starting at the nucleus and increasing outwards (1, 2, 3...). The maximum number of electrons for any shell is given by the formula 2n2 where n stands for the shell number ...
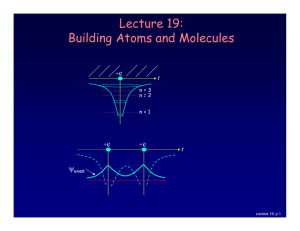
Lecture 19: Building Atoms and Molecules
... It’s more like filling a bucket with water. • They are distributed among the energy levels according to the Exclusion Principle. • Particles that obey this principle are called “fermions”. Protons and neutrons are also fermions, but photons are not. Lecture 19, p 10 ...
... It’s more like filling a bucket with water. • They are distributed among the energy levels according to the Exclusion Principle. • Particles that obey this principle are called “fermions”. Protons and neutrons are also fermions, but photons are not. Lecture 19, p 10 ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.