The Bohr model depicts atoms as small, positively
... Although it challenged the knowledge of classical physics, the model's success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was intro ...
... Although it challenged the knowledge of classical physics, the model's success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was intro ...
Nature template - PC Word 97
... An atom in the momentum state |-kL> can be reflected to the state |+kL> by a standing wave laser pulse. This is a process called Bragg diffration. After a time T0 = 2 ħk/mg≈1.2 ms for 87Rb, the state |+kL> evolves back into the state |-kL> because of the downwards acceleration of gravity g. Repeatin ...
... An atom in the momentum state |-kL> can be reflected to the state |+kL> by a standing wave laser pulse. This is a process called Bragg diffration. After a time T0 = 2 ħk/mg≈1.2 ms for 87Rb, the state |+kL> evolves back into the state |-kL> because of the downwards acceleration of gravity g. Repeatin ...
Chapter 7 Covalent Bonding Outline Covalent Bonding Introduction
... 1. Count the number of valence electrons 2. Draw a skeleton structure for the species, joining the atoms by single bonds 3. Determine the number of valence electrons still available for distribution 4. Determine the number of valence electrons required to fill out an octet for each atom (except H) i ...
... 1. Count the number of valence electrons 2. Draw a skeleton structure for the species, joining the atoms by single bonds 3. Determine the number of valence electrons still available for distribution 4. Determine the number of valence electrons required to fill out an octet for each atom (except H) i ...
Atomic Physics
... to be something like 1s 3 , since this would imply that two electrons would have to share the same ms number (i.e., the ending superscript cannot be greater than two for a ns orbital). To minimize energy two electrons will still occupy the inner shell 1s 2 , while the third one will reside on the ne ...
... to be something like 1s 3 , since this would imply that two electrons would have to share the same ms number (i.e., the ending superscript cannot be greater than two for a ns orbital). To minimize energy two electrons will still occupy the inner shell 1s 2 , while the third one will reside on the ne ...
HW / Unit 2
... 6. Place the following atoms in order of increasing size: S, Rb, K, C, O, Al, P 7. What happens to the size of an atom when it loses an electron? Gains an electron? 8. Place the following atoms and ions in order of increasing size: Cl, Cl-, Mg, Mg2+ 9. Which element is the most common in the univers ...
... 6. Place the following atoms in order of increasing size: S, Rb, K, C, O, Al, P 7. What happens to the size of an atom when it loses an electron? Gains an electron? 8. Place the following atoms and ions in order of increasing size: Cl, Cl-, Mg, Mg2+ 9. Which element is the most common in the univers ...
Wave-particle_duality
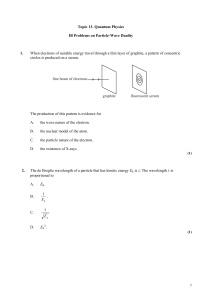
... An electron of mass me and a proton of mass mp are moving with the same speed. The de Broglie wavelengths associated with the electron and with the proton are e and p respectively. The ratio ...
... An electron of mass me and a proton of mass mp are moving with the same speed. The de Broglie wavelengths associated with the electron and with the proton are e and p respectively. The ratio ...
Midterm Review Packet - Mrs. McKenzie`s Chemistry and ICP Classes
... 10. In an ionic bond, __________ atoms of ________________ charge are held together by _________________________ attraction. 11. The part of an atom that has a neutral charge is a _______________________. 12. Most of the mass of an atom is found in the _____________________. 13. A pure substance mad ...
... 10. In an ionic bond, __________ atoms of ________________ charge are held together by _________________________ attraction. 11. The part of an atom that has a neutral charge is a _______________________. 12. Most of the mass of an atom is found in the _____________________. 13. A pure substance mad ...
Single-photon multiple ionization processes studied by electron coincidence spectroscopy Per Linusson
... peaks in photoelectron spectra, side bands were also observed that could only be accounted for if electron-electron interactions were taken into account in the physical description in a more refined way than in the independent particle methods (see e.g. Ref. [10] and references therein). In some cas ...
... peaks in photoelectron spectra, side bands were also observed that could only be accounted for if electron-electron interactions were taken into account in the physical description in a more refined way than in the independent particle methods (see e.g. Ref. [10] and references therein). In some cas ...
CHEM 481. Assignment 0. Review of General Chemistry. Answers
... endothermic process, since bonds form by releasing energy, so it costs energy to break them. 54. What is the relationship between bond order, bond length, and bond energy for a series of related bonds, say carbonnitrogen bonds? Higher bond order has higher bond energy and shorter bond length. 55. If ...
... endothermic process, since bonds form by releasing energy, so it costs energy to break them. 54. What is the relationship between bond order, bond length, and bond energy for a series of related bonds, say carbonnitrogen bonds? Higher bond order has higher bond energy and shorter bond length. 55. If ...
Answers to Assignment #1
... endothermic process, since bonds form by releasing energy, so it costs energy to break them. 54. What is the relationship between bond order, bond length, and bond energy for a series of related bonds, say carbonnitrogen bonds? Higher bond order has higher bond energy and shorter bond length. 55. If ...
... endothermic process, since bonds form by releasing energy, so it costs energy to break them. 54. What is the relationship between bond order, bond length, and bond energy for a series of related bonds, say carbonnitrogen bonds? Higher bond order has higher bond energy and shorter bond length. 55. If ...
Outline Ch 8 - Mead`s Fabulous Weebly
... A. 3D Structure of molecules Electron dot diagrams only give a flat view of the molecule (in 2D) Real molecules are not flat but 3D VSEPR theory explains and predicts the 3D shape of molecules Valence Shell Electron Pair Repulsion Theory Electron pairs repulse each other Shape will be ba ...
... A. 3D Structure of molecules Electron dot diagrams only give a flat view of the molecule (in 2D) Real molecules are not flat but 3D VSEPR theory explains and predicts the 3D shape of molecules Valence Shell Electron Pair Repulsion Theory Electron pairs repulse each other Shape will be ba ...
ON THE SHAPES OF ATOMS
... Contradictory ideas appear, however, in other open-shell situations. For example, it is not uncommon to find the shape of an atom having only one electron in the outer p sub-shell regarded as arising from the superposition of a spherical distribution and a 2p (2p x , say) dumbell-like cloud, and tha ...
... Contradictory ideas appear, however, in other open-shell situations. For example, it is not uncommon to find the shape of an atom having only one electron in the outer p sub-shell regarded as arising from the superposition of a spherical distribution and a 2p (2p x , say) dumbell-like cloud, and tha ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.