che-20028 QC lecture 2 - Rob Jackson`s Website
... crystals (why?), so they can be used to study the surfaces of crystals. • This effect is exploited in low energy electron diffraction, where, provided the energies are low enough, surface features like adsorbed molecules can be detected (important in catalysis). CHE-20028 QC lecture 2 ...
... crystals (why?), so they can be used to study the surfaces of crystals. • This effect is exploited in low energy electron diffraction, where, provided the energies are low enough, surface features like adsorbed molecules can be detected (important in catalysis). CHE-20028 QC lecture 2 ...
Quantum Physics 3 - FSU Physics Department
... Stern-Gerlach results cannot be explained by interaction of magnetic moment from orbital angular momentum must be due to some additional internal source of angular momentum that does not require motion of the electron. internal angular momentum of electron (“spin”) was suggested in 1925 by Gou ...
... Stern-Gerlach results cannot be explained by interaction of magnetic moment from orbital angular momentum must be due to some additional internal source of angular momentum that does not require motion of the electron. internal angular momentum of electron (“spin”) was suggested in 1925 by Gou ...
Pulsed field ionization of Rydberg atoms
... atom that is kicked by a pulsed electric field. The two calculations are compared to recent experimental results through the ionization probability versus peak field strength. The energy distributions of the final electrons are compared between the two calculations. There is a qualitative difference ...
... atom that is kicked by a pulsed electric field. The two calculations are compared to recent experimental results through the ionization probability versus peak field strength. The energy distributions of the final electrons are compared between the two calculations. There is a qualitative difference ...
The AC Stark effect - Center for Ultracold Atoms
... Consider a laser of frequency ω and intensity I which creates a dipole trap for an atom. a) Consider a two-level system with lower state |Si and upper state |P i with ‘bare’ energy separation ~ωP S . Also let the dipole moment be dP S . i. Find the AC Stark shift for the state |Si in terms of the di ...
... Consider a laser of frequency ω and intensity I which creates a dipole trap for an atom. a) Consider a two-level system with lower state |Si and upper state |P i with ‘bare’ energy separation ~ωP S . Also let the dipole moment be dP S . i. Find the AC Stark shift for the state |Si in terms of the di ...
Esters of Nitric Acid as Electron Acceptors
... are charge transfer complexes, and which of the components is the electron acceptor. A suggestion was advanced that nitric esters can act as electron acceptors and this was substantiated by our present work. We examined now an interaction between nitric esters of mono-, di-, tri-, tetraand hexahydro ...
... are charge transfer complexes, and which of the components is the electron acceptor. A suggestion was advanced that nitric esters can act as electron acceptors and this was substantiated by our present work. We examined now an interaction between nitric esters of mono-, di-, tri-, tetraand hexahydro ...
Lecture 7
... •The total energy of a planet only depends on its orbital radius (higher energy, bigger radius); the same should be true of the electrons in an atom. ...
... •The total energy of a planet only depends on its orbital radius (higher energy, bigger radius); the same should be true of the electrons in an atom. ...
SAMPLE midterm with solutions
... sides of the sample, which carry current in one direction only and are in separate equilibrium. The states on a single edge are chiral, that is, they propagate only in one direction. Therefore even if an electron scatters at the edge, this does not degrade the current. Because the edge states are se ...
... sides of the sample, which carry current in one direction only and are in separate equilibrium. The states on a single edge are chiral, that is, they propagate only in one direction. Therefore even if an electron scatters at the edge, this does not degrade the current. Because the edge states are se ...
2 Chemical bonding is a genuinely quantum effect, which cannot be
... • Hybridization: clearly, an sp3 carbon with four single bonds differs from an sp2 carbon with a double bond or even an sp carbon with a triple bond. So, we need different potentials for the C–C, C=C and C≡C bonds. Also, there will be various different C atom types as well, like an aromatic carbon e ...
... • Hybridization: clearly, an sp3 carbon with four single bonds differs from an sp2 carbon with a double bond or even an sp carbon with a triple bond. So, we need different potentials for the C–C, C=C and C≡C bonds. Also, there will be various different C atom types as well, like an aromatic carbon e ...
IO-IY
... high-voltage discharge tube. This transition lies in the visible region (380-7S0 nm) of the hydrogen spectrum. Thus, we can conclude that the transition is in the Balmer series and, therefore, that nf = 2. The value of ni can be found using Equation 30.14, according to which the Ba1mer series transi ...
... high-voltage discharge tube. This transition lies in the visible region (380-7S0 nm) of the hydrogen spectrum. Thus, we can conclude that the transition is in the Balmer series and, therefore, that nf = 2. The value of ni can be found using Equation 30.14, according to which the Ba1mer series transi ...
Chapter 2 Chemical context of Life
... An orbital is a three-dimensional space where the electron is found 90% of the time. An orbital contains a maximum of two electrons. Electrons in orbitals with similar energies occupy the same principal energy level. The orbitals of an energy level are designated by the letters s, p, d and f. See fi ...
... An orbital is a three-dimensional space where the electron is found 90% of the time. An orbital contains a maximum of two electrons. Electrons in orbitals with similar energies occupy the same principal energy level. The orbitals of an energy level are designated by the letters s, p, d and f. See fi ...
REsults
... We measured PL and PLE spectra of QWR with various gate voltages from 0 to 0.7V at 5K. Fig.2 (a) shows the normalized PL (dotted lines) and PLE (solid lines) spectra, where PL excitation energy is adjusted between 1.569 eV and 1.575eV. At low electron density (0V), the PLE spectrum is dominated by t ...
... We measured PL and PLE spectra of QWR with various gate voltages from 0 to 0.7V at 5K. Fig.2 (a) shows the normalized PL (dotted lines) and PLE (solid lines) spectra, where PL excitation energy is adjusted between 1.569 eV and 1.575eV. At low electron density (0V), the PLE spectrum is dominated by t ...
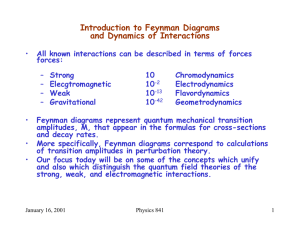
Introduction to Feynman Diagrams and Dynamics of Interactions
... perturbation theory in nonrelativistic quantum mechanics, we have second order perturbation theory in quantum field theories. ...
... perturbation theory in nonrelativistic quantum mechanics, we have second order perturbation theory in quantum field theories. ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... Explaining the MOT with a different notation The quantum axis is now not an arbitrary axis in space like before but parallel to the local magnetic field or the photon propagation direction. Let look at the angular momentum conservation in a simplified J=1/2 system. Magnetic field in the MOT ...
... Explaining the MOT with a different notation The quantum axis is now not an arbitrary axis in space like before but parallel to the local magnetic field or the photon propagation direction. Let look at the angular momentum conservation in a simplified J=1/2 system. Magnetic field in the MOT ...
3 Radiation processes 3.1 Atomic and molecular structure
... (4π/3)re . In quantum systems, the upper layer could decay through different channels; then the profile of each line is also described by the Lorentz profile with an appropriate ν0 but the line width γ is determined by the total decay probability for the level. If only radiation transitions are poss ...
... (4π/3)re . In quantum systems, the upper layer could decay through different channels; then the profile of each line is also described by the Lorentz profile with an appropriate ν0 but the line width γ is determined by the total decay probability for the level. If only radiation transitions are poss ...
slides in pdf format
... • Because the energy is so certain it means something else must be very uncertain • Example: the location of an electron in the atom. Any one measurement will find an electron in the atom at some particular point - the theory only predicts the probability of finding the electron at any point – we ca ...
... • Because the energy is so certain it means something else must be very uncertain • Example: the location of an electron in the atom. Any one measurement will find an electron in the atom at some particular point - the theory only predicts the probability of finding the electron at any point – we ca ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.