Chapter 4: Solution Chemistry and the Hydrosphere
... Oxidation Number (or Oxidation State): actual or hypothetical charge of an atom in a compound if it existed as a monatomic ion ...
... Oxidation Number (or Oxidation State): actual or hypothetical charge of an atom in a compound if it existed as a monatomic ion ...
I - Holland Public Schools
... * chemical kinetics - the study of rates of chemical reactions * examples: ...
... * chemical kinetics - the study of rates of chemical reactions * examples: ...
Electrochemistry
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Decomposition Reactions
... Starting from this classification scheme, reactions can be further classified according to the type of chemistry that occurs – acid-base neutralization, or oxidation-reduction reactions, for example. In this lab activity, you will investigate one example of a decomposition reaction. This type of rea ...
... Starting from this classification scheme, reactions can be further classified according to the type of chemistry that occurs – acid-base neutralization, or oxidation-reduction reactions, for example. In this lab activity, you will investigate one example of a decomposition reaction. This type of rea ...
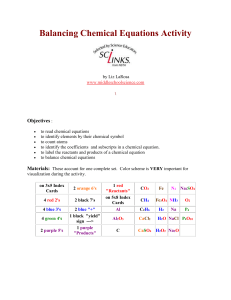
Balancing Chemical Equations Activity by Liz LaRosa www
... Identify the elements on the reactant side. Count the number of atoms for each element. Identify the elements on the product side. Count the number of atoms on the product side. Are the 2 sides equal? If not, the equation is not balanced. The index cards numbered 2 - 7 are your coefficients. They ca ...
... Identify the elements on the reactant side. Count the number of atoms for each element. Identify the elements on the product side. Count the number of atoms on the product side. Are the 2 sides equal? If not, the equation is not balanced. The index cards numbered 2 - 7 are your coefficients. They ca ...
The Relation between Salt and Ionic Transport Coefficients
... in (c) largely cancel each other. Thus, for example, if the solutions contain NaC1 at 0.1 and 0.01 M, respectively, the values for ~ according to the two definitions agree within 2 %. If, however, the lower concentration is decreased to a very small value, the last term in (e) vanishes while the oth ...
... in (c) largely cancel each other. Thus, for example, if the solutions contain NaC1 at 0.1 and 0.01 M, respectively, the values for ~ according to the two definitions agree within 2 %. If, however, the lower concentration is decreased to a very small value, the last term in (e) vanishes while the oth ...
chem equation Pkt Student2
... 1) Which side of the yields arrow do you find reactants? ______________________________ 2) Which side of the yields arrow do you find products? _______________________________ 3) In a chemical equation, what do the coefficients represent? ______________________________ 4) In a chemical equation, wha ...
... 1) Which side of the yields arrow do you find reactants? ______________________________ 2) Which side of the yields arrow do you find products? _______________________________ 3) In a chemical equation, what do the coefficients represent? ______________________________ 4) In a chemical equation, wha ...
Chemical properties of amines:
... Organic chemistry and Biological chemistry for Health Sciences ...
... Organic chemistry and Biological chemistry for Health Sciences ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.