Computer simulation of the dynamics of aqueous solvation
... the basis of the vast amount already known about aqueous solvation. 31 Especially in terms of computer simulations, the number of studies of aqueous solvation is at least an order of magnitude greater than for all other solvents combined. Traditionally, simulations have mainly concerned the equilibr ...
... the basis of the vast amount already known about aqueous solvation. 31 Especially in terms of computer simulations, the number of studies of aqueous solvation is at least an order of magnitude greater than for all other solvents combined. Traditionally, simulations have mainly concerned the equilibr ...
Chapter 2 The Electroless Nickel Plating Bath: Effect of Variables on
... discussed from the viewpoint of the function each performs in the bath, with little attention paid to their effect on the plating process. The metal and the electron source (the reducing agent) are consumed in the electroless plating reaction and so their concentrations in the bath are continuously ...
... discussed from the viewpoint of the function each performs in the bath, with little attention paid to their effect on the plating process. The metal and the electron source (the reducing agent) are consumed in the electroless plating reaction and so their concentrations in the bath are continuously ...
Chemistry Final Exam Review
... c. high pressure and high temperature b. low pressure and high temperature d. high pressure and low temperature ____ 136. When does a real gas behave like an ideal gas? a. when the particles are far apart b. when the kinetic energy of the particles is low c. when the pressure is high d. when the gas ...
... c. high pressure and high temperature b. low pressure and high temperature d. high pressure and low temperature ____ 136. When does a real gas behave like an ideal gas? a. when the particles are far apart b. when the kinetic energy of the particles is low c. when the pressure is high d. when the gas ...
1.09 MB / 64 pages
... Whether you can predict that a substance will be soluble in water by looking at its line formula depends to some extent on how the formula is actually written. For example, the line formula C2H6O, does not tell you how the atoms are connected, so the best you can do is predict that the molecule will ...
... Whether you can predict that a substance will be soluble in water by looking at its line formula depends to some extent on how the formula is actually written. For example, the line formula C2H6O, does not tell you how the atoms are connected, so the best you can do is predict that the molecule will ...
Improved Synthesis of Seven-Coordinate Molybdenum( I I) and
... immediately with concomitant gas evolution. This solution was heated to reflux for 8 h. The solvent was removed under reduced pressure and replaced with 250 mL of absolute ethanol. The bright orange19solution was purged with nitrogen for 30 min and then photolyzed with a Pyrex-jacketed 450-Watt medi ...
... immediately with concomitant gas evolution. This solution was heated to reflux for 8 h. The solvent was removed under reduced pressure and replaced with 250 mL of absolute ethanol. The bright orange19solution was purged with nitrogen for 30 min and then photolyzed with a Pyrex-jacketed 450-Watt medi ...
Laboratory Works and Home Tasks in General Chemistry
... the equivalent (in moles) in one liter of the solution. It is defined as CN (sometimes N) and is calculated as a ratio of the amount of the equivalent of the dissolved substance X to the volume of the solution in liters: CN(X) = neq(X) / V. Having expressed in the denominator the molar mass of the e ...
... the equivalent (in moles) in one liter of the solution. It is defined as CN (sometimes N) and is calculated as a ratio of the amount of the equivalent of the dissolved substance X to the volume of the solution in liters: CN(X) = neq(X) / V. Having expressed in the denominator the molar mass of the e ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... and +4 states are strongly oxidising, while the Rh(III) state is the most stable. The rhodium(I) oxidation state has a d8 electron configuration and usually occurs in four-coordinate square planar structures e.g. [Rh(CO)Cl(PCy3)2] or five-coordinate trigonal bipyrimidal structures3 e.g. [HRh(PF3)4]. ...
... and +4 states are strongly oxidising, while the Rh(III) state is the most stable. The rhodium(I) oxidation state has a d8 electron configuration and usually occurs in four-coordinate square planar structures e.g. [Rh(CO)Cl(PCy3)2] or five-coordinate trigonal bipyrimidal structures3 e.g. [HRh(PF3)4]. ...
Chemistry In action
... This book was typeset in 10/12 Times New Roman at cMPreparé and printed and bound by R. R. Donnelley/Jefferson City. The cover was printed by R. R. Donnelley/Jefferson City. The paper in this book was manufactured by a mill whose forest management programs include sustained yield—harvesting of its t ...
... This book was typeset in 10/12 Times New Roman at cMPreparé and printed and bound by R. R. Donnelley/Jefferson City. The cover was printed by R. R. Donnelley/Jefferson City. The paper in this book was manufactured by a mill whose forest management programs include sustained yield—harvesting of its t ...
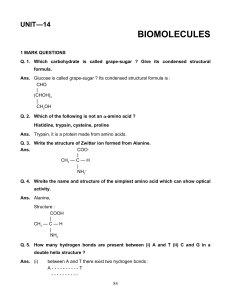
HOTS Worksheet
... Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble gum or chewing gum ? Ans. Bubble gum or chewing gum contains ...
... Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble gum or chewing gum ? Ans. Bubble gum or chewing gum contains ...
Chapter 4 - AP Chemistry with dr hart
... Practice Exercise 2 (4.6) Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO3)2 (calcium nitrate), C6H12O6 (glucose), NaC2H3O2 (sodium acetate), and HC2H3O2 (acetic acid). Rank the solutions in order of increasing electrical conductivity, base ...
... Practice Exercise 2 (4.6) Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO3)2 (calcium nitrate), C6H12O6 (glucose), NaC2H3O2 (sodium acetate), and HC2H3O2 (acetic acid). Rank the solutions in order of increasing electrical conductivity, base ...
Chapter 3 Stoichiometry: Calculations with Chemical
... Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
... Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Find the empirical formula of PABA. ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.