Thermochemical Investigations of Nearly Ideal
... allow compensation for the effects of solution nonideality, or, from a slightly different viewpoint, to separate "chemical" and "physical" effects on the properties of the complexes. In order to provide a firm thermodynamic basis for these approximations, much simpler systems must be studied, establ ...
... allow compensation for the effects of solution nonideality, or, from a slightly different viewpoint, to separate "chemical" and "physical" effects on the properties of the complexes. In order to provide a firm thermodynamic basis for these approximations, much simpler systems must be studied, establ ...
Chemistry Worksheets
... 1) A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density? 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. 3) What is the w ...
... 1) A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density? 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. 3) What is the w ...
A Few Things You Might Want To Know
... Law of Definite Proportions (Law of Constant Composition) The composition of a compound is constant. The relative masses of elements in a compound form simple, whole-number ratios. ...
... Law of Definite Proportions (Law of Constant Composition) The composition of a compound is constant. The relative masses of elements in a compound form simple, whole-number ratios. ...
2015 International Practice Exam: Chemistry
... You will now take the multiple-choice portion of the exam. You should have in front of you the multiple-choice booklet and your answer sheet. You may never discuss these specific multiple-choice questions at any time in any form with anyone, including your teacher and other students. If you disclose ...
... You will now take the multiple-choice portion of the exam. You should have in front of you the multiple-choice booklet and your answer sheet. You may never discuss these specific multiple-choice questions at any time in any form with anyone, including your teacher and other students. If you disclose ...
11.1 Enthalpy PowerPoint
... Theoretically, we assume that the enthalpy change of a physical or chemical process depends only on the initial and final conditions. It is independent of the pathway, process or number of intermediate steps required. ...
... Theoretically, we assume that the enthalpy change of a physical or chemical process depends only on the initial and final conditions. It is independent of the pathway, process or number of intermediate steps required. ...
PRACTICE EXAM 1-C
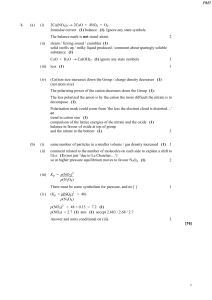
... A yellow precipitate of silver phosphate, Ag3PO4, is formed in a double displacement reaction. a) ...
... A yellow precipitate of silver phosphate, Ag3PO4, is formed in a double displacement reaction. a) ...
Separation of Magnesium Hydroxide and Barium Sulphate from a
... In the previous section it was shown that Mg(OH)2 can be dissolved by the formation of Mg(HCO3)2, through CO2 addition without affecting the low solubility of BaSO4. The sludge separation process will be more effective at higher solubility values for Mg(HCO3)2. Therefore, it was decided to determine ...
... In the previous section it was shown that Mg(OH)2 can be dissolved by the formation of Mg(HCO3)2, through CO2 addition without affecting the low solubility of BaSO4. The sludge separation process will be more effective at higher solubility values for Mg(HCO3)2. Therefore, it was decided to determine ...
UNIVERSITI MALAYSIA SABAH
... Aluminium occurs widely in nature as aluminosilicate minerals and as bauxite, Al2O3.xH2O from which the metal can be produced by electrolysis after dissolving in molten cryolite, Na3AlF6. The metal is mainly used in aluminium alloys. The organoaluminium compounds (e.g., Et3Al) are used in the cataly ...
... Aluminium occurs widely in nature as aluminosilicate minerals and as bauxite, Al2O3.xH2O from which the metal can be produced by electrolysis after dissolving in molten cryolite, Na3AlF6. The metal is mainly used in aluminium alloys. The organoaluminium compounds (e.g., Et3Al) are used in the cataly ...
Homogeneous Catalysis
... Chemical Kinetics is the study of reaction rates; that is, how fast a given reaction does proceeds. It is a measure of the change of the concentration of reactants (or products) as a function of time. Reaction rates provide information regarding how fast a chemical process occurs as well as the mech ...
... Chemical Kinetics is the study of reaction rates; that is, how fast a given reaction does proceeds. It is a measure of the change of the concentration of reactants (or products) as a function of time. Reaction rates provide information regarding how fast a chemical process occurs as well as the mech ...
Learning Outcomes Leaving Certificate Chemistry
... define relative atomic mass (Ar) using the C12 scale define isotope describe the composition of isotopes using hydrogen and carbon as examples describe how a mass spectrometer can be used to determine relative atomic mass describe the principles on which the Mass Spectrometer is based explain the fu ...
... define relative atomic mass (Ar) using the C12 scale define isotope describe the composition of isotopes using hydrogen and carbon as examples describe how a mass spectrometer can be used to determine relative atomic mass describe the principles on which the Mass Spectrometer is based explain the fu ...
Stoichiometric Problems III: Sto c o et c ob e s
... Here is a typical problem. problem If I have the following chemical reaction: N2(g) + 3H2 (g) = 2NH3 (g) And I start with 5 moles of N2 and 5 moles of H2, which chemical (H2 or N2) is the limiting reactant? My approach to solving this kind of problem is to calculate the amount of product that would ...
... Here is a typical problem. problem If I have the following chemical reaction: N2(g) + 3H2 (g) = 2NH3 (g) And I start with 5 moles of N2 and 5 moles of H2, which chemical (H2 or N2) is the limiting reactant? My approach to solving this kind of problem is to calculate the amount of product that would ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.