Thermochemistry - Ars
... This gives a unit of energy as being kg m2 s-2 . This unit is the SI unit of energy. It has been renamed the Joule (J) in honor of James Prescott Joule who did some of the earlier work on energy transfer and heat. Another unit of energy that has been used and is still in use in some places, like nut ...
... This gives a unit of energy as being kg m2 s-2 . This unit is the SI unit of energy. It has been renamed the Joule (J) in honor of James Prescott Joule who did some of the earlier work on energy transfer and heat. Another unit of energy that has been used and is still in use in some places, like nut ...
Chapter 4 Packet
... 5. predict the products of chemical reactions (including neutralization and precipitation reactions) and write balanced molecular and net ionic equations for them. I will also be able identify spectator ions. 6. be able to choose which type of equation is most appropriate (molecular, ionic or net io ...
... 5. predict the products of chemical reactions (including neutralization and precipitation reactions) and write balanced molecular and net ionic equations for them. I will also be able identify spectator ions. 6. be able to choose which type of equation is most appropriate (molecular, ionic or net io ...
Міністерство охорони здоров`я України
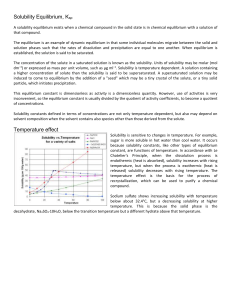
... vessels, and trigger harmful chemical reactions in the blood. Supersevere conditions are observed at the cerebral vascular occlusion. The solubility of gases in liquids depends on the nature of the gas, and the nature of the solvent, complying the rule "like dissolves like". ...
... vessels, and trigger harmful chemical reactions in the blood. Supersevere conditions are observed at the cerebral vascular occlusion. The solubility of gases in liquids depends on the nature of the gas, and the nature of the solvent, complying the rule "like dissolves like". ...
lect1f
... quantities, if they are related to unit mass, volume, etc. Density = m/V Molar volume: Vm = V/n (subscript m refer to molar) Molar internal energy: Um = U/n Equation of state: is a relationship among the state variables of the system in equilibrium . ...
... quantities, if they are related to unit mass, volume, etc. Density = m/V Molar volume: Vm = V/n (subscript m refer to molar) Molar internal energy: Um = U/n Equation of state: is a relationship among the state variables of the system in equilibrium . ...
The collision theory of reactions
... In the Haber process, (reaction between H2 and N2) at 300 K only 1 in 1011 collisions between H2 and N2 results in a reaction! Even at 800 K only 1 in 104 collisions results in a reaction. The collision theory says: Reactions occur when molecules collide with a certain minimum kinetic energy. The mo ...
... In the Haber process, (reaction between H2 and N2) at 300 K only 1 in 1011 collisions between H2 and N2 results in a reaction! Even at 800 K only 1 in 104 collisions results in a reaction. The collision theory says: Reactions occur when molecules collide with a certain minimum kinetic energy. The mo ...
CHEM1001 2012-J-2 June 2012 22/01(a) • Complete the following
... Water is a dipolar molecule. The positive ends (H) can directly interact with negative ions, while the negative end (O) can directly interact with positive ions. These interactions (called hydration enthalpy) are often sufficient to overcome the lattice enthalpy (the energy required to break up the ...
... Water is a dipolar molecule. The positive ends (H) can directly interact with negative ions, while the negative end (O) can directly interact with positive ions. These interactions (called hydration enthalpy) are often sufficient to overcome the lattice enthalpy (the energy required to break up the ...
Acid-Base Equilibria and Activity
... strong acid and strong base solutions. Determine the limiting reagent when each pair of solutions are mixed. From this result you can also determine if the resulting solution is acidic or neutral and calculate the pH (pH = − log[H+ ]). Use the example above as a guide. ...
... strong acid and strong base solutions. Determine the limiting reagent when each pair of solutions are mixed. From this result you can also determine if the resulting solution is acidic or neutral and calculate the pH (pH = − log[H+ ]). Use the example above as a guide. ...
CHEM 150
... ____ 23. Which of the following molecules can have only London dispersion forces? a. CH4 b. CO2 c. both (a) and (b) d. neither (a) nor (b) ____ 24. Which of the following molecules cannot engage in hydrogen bonding? a. CH4 b. NH3 c. H2O d. all of them ____ 25. When comparing a liquid with a gas at t ...
... ____ 23. Which of the following molecules can have only London dispersion forces? a. CH4 b. CO2 c. both (a) and (b) d. neither (a) nor (b) ____ 24. Which of the following molecules cannot engage in hydrogen bonding? a. CH4 b. NH3 c. H2O d. all of them ____ 25. When comparing a liquid with a gas at t ...
Chemical Equilibrium - 2012 Book Archive
... 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it wa ...
... 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it wa ...
Chapter 4 - Aqueous Reactions
... Note that the equation is balanced for both mass and charge!!! ...
... Note that the equation is balanced for both mass and charge!!! ...
2017 Chemistry Exam Review Compounds and Reactions 1. Know
... 39. Draw water molecules to show how, based on their polarity, they cling together. What properties of water does this “clinginess” cause? 40. Draw water molecules to show how, based on their polarity, they dissolve table salt (NaCl) by surrounding Na+ cations and Cl- anions. 41. What kind of substa ...
... 39. Draw water molecules to show how, based on their polarity, they cling together. What properties of water does this “clinginess” cause? 40. Draw water molecules to show how, based on their polarity, they dissolve table salt (NaCl) by surrounding Na+ cations and Cl- anions. 41. What kind of substa ...
Final Exam Study Guide Chapters 1-12
... ____ 55. In metallic bonds, the mobile electrons surrounding the positive ions are called a(n) a. Lewis structure. c. electron cloud. b. electron sea. d. dipole. ____ 56. To appear shiny, a material must be able to a. form crystals. b. absorb and re-emit light of many wavelengths. c. absorb light an ...
... ____ 55. In metallic bonds, the mobile electrons surrounding the positive ions are called a(n) a. Lewis structure. c. electron cloud. b. electron sea. d. dipole. ____ 56. To appear shiny, a material must be able to a. form crystals. b. absorb and re-emit light of many wavelengths. c. absorb light an ...
Mass-Mass Stoichiometry
... (Rn) is 86. Write the equation for this process 31. Suppose you have a radioactive isotope with a half-life of 2 years and you start with 800 grams of this substance today. How long will it take before you have 100 grams of this substance? How long will it take before you have 25 grams? 32. Write th ...
... (Rn) is 86. Write the equation for this process 31. Suppose you have a radioactive isotope with a half-life of 2 years and you start with 800 grams of this substance today. How long will it take before you have 100 grams of this substance? How long will it take before you have 25 grams? 32. Write th ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.