ch14 lecture 7e
... Zeff increases for the larger 3A elements due to poor shielding by d and f electrons. The larger 3A elements have smaller atomic radii and larger ionization energies and electronegativities than expected. These properties influence the physical and chemical behavior of these elements. ...
... Zeff increases for the larger 3A elements due to poor shielding by d and f electrons. The larger 3A elements have smaller atomic radii and larger ionization energies and electronegativities than expected. These properties influence the physical and chemical behavior of these elements. ...
Biochem
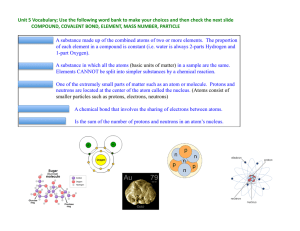
... • The number of protons in an atom is equal to the number of electrons. • Consequently, the number of positive charges equals the number of negative charges, resulting in a net charge of zero. • Therefore, atoms do not have a charge and are considered to be neutral. ...
... • The number of protons in an atom is equal to the number of electrons. • Consequently, the number of positive charges equals the number of negative charges, resulting in a net charge of zero. • Therefore, atoms do not have a charge and are considered to be neutral. ...
atoms
... ago, scientists found that certain types of matter couldn’t be broken down into any other simpler substances They called these special pure substances: elements ...
... ago, scientists found that certain types of matter couldn’t be broken down into any other simpler substances They called these special pure substances: elements ...
Atoms - Chemistry1Advanced
... masses of atoms are so small, it is more convenient to use relative atomic masses instead of real masses to set up a scale, we have to pick one atom to be the standard since 1961, the carbon-12 nuclide is the standard and is assigned a mass of ...
... masses of atoms are so small, it is more convenient to use relative atomic masses instead of real masses to set up a scale, we have to pick one atom to be the standard since 1961, the carbon-12 nuclide is the standard and is assigned a mass of ...
200 Ways to Pass the Chemistry
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl It loses its 1 valence electron leaving 2 below it 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are fo ...
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl It loses its 1 valence electron leaving 2 below it 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are fo ...
Review Unit 5
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
Atomic Radii
... Why do sodium ions have a smaller radii than sodium atoms What is the definition of the term. Where are the atoms located on the periodic table (and valence electrons). How this affects the stated trend. Why it affects the stated trend and relate back to question. Sodium atom and sodium ions have th ...
... Why do sodium ions have a smaller radii than sodium atoms What is the definition of the term. Where are the atoms located on the periodic table (and valence electrons). How this affects the stated trend. Why it affects the stated trend and relate back to question. Sodium atom and sodium ions have th ...
Ch. 10 – Stoichiometry Stoichiometry – relates molar ratios between
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
Atoms of an element are identical
... _____4. According to the law of conservation of mass, when sodium, hydrogen, and oxygen react to form a compound, the mass of the compound is _________ the sum of the masses of the individual elements. a. Equal to. b. Greater than. c. Less than. d. Either greater than or less than. _____5. Accordin ...
... _____4. According to the law of conservation of mass, when sodium, hydrogen, and oxygen react to form a compound, the mass of the compound is _________ the sum of the masses of the individual elements. a. Equal to. b. Greater than. c. Less than. d. Either greater than or less than. _____5. Accordin ...
ch22_lecture_6e_final
... – The inorganic cycle involves slow weathering of phosphatecontaining rocks, which causes PO43- to leach into the rivers and seas. – The land-based biological cycle involves incorporation of PO43- into organisms and its release through excretion and ...
... – The inorganic cycle involves slow weathering of phosphatecontaining rocks, which causes PO43- to leach into the rivers and seas. – The land-based biological cycle involves incorporation of PO43- into organisms and its release through excretion and ...
Total Notes for chem - Catawba County Schools
... based on these ideas; 1. When elements combine, the outermost electrons; that is, those electrons with levels of energy which will cause them to orbit at the greatest distance from the nucleus, will be the only electrons directly involved with the reaction of these elements to form compounds. 2. The ...
... based on these ideas; 1. When elements combine, the outermost electrons; that is, those electrons with levels of energy which will cause them to orbit at the greatest distance from the nucleus, will be the only electrons directly involved with the reaction of these elements to form compounds. 2. The ...
Thomson`s Atomic Model
... is made of atoms, should be negatively charged as well. • But matter is not negatively charge. • Atoms must also contain some positive charge? ...
... is made of atoms, should be negatively charged as well. • But matter is not negatively charge. • Atoms must also contain some positive charge? ...
§2 Atomic Structure , A website that gives a good summary of this
... Isotopes of an element have the same number of protons (and electrons) but have different numbers of neutrons, common isotope of carbon, C-12, has 6 protons in its nucleus and also 6 neutrons while the rarer C-14 contains 6 The chemical properties of isotopes are very similar due to the equal number ...
... Isotopes of an element have the same number of protons (and electrons) but have different numbers of neutrons, common isotope of carbon, C-12, has 6 protons in its nucleus and also 6 neutrons while the rarer C-14 contains 6 The chemical properties of isotopes are very similar due to the equal number ...
File - Biochemistry
... Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic masses and relative abundances as follows: 23.985 amu (78.99%) 24.986 amu (10.00%) 25.982 amu (11.01%) ...
... Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic masses and relative abundances as follows: 23.985 amu (78.99%) 24.986 amu (10.00%) 25.982 amu (11.01%) ...
ATOMS: THE BUILDING BLOCKS OF MATTER from the
... Hund's Rule of Maximum Multiplicity Electrons fill the energy levels in regular patterns. They first fill the s orbitals, then the p, and so on. hydrogen - The element with the atomic number of one and symbol of H. Hydrogen is the most common element in the universe. ion - An atom with more or less ...
... Hund's Rule of Maximum Multiplicity Electrons fill the energy levels in regular patterns. They first fill the s orbitals, then the p, and so on. hydrogen - The element with the atomic number of one and symbol of H. Hydrogen is the most common element in the universe. ion - An atom with more or less ...
Chapter 2 Chemical Basis of Life
... Similarities of elements within a column occur because they have the same number of electrons in their outer shells, and therefore they have similar chemical bonding properties ...
... Similarities of elements within a column occur because they have the same number of electrons in their outer shells, and therefore they have similar chemical bonding properties ...
An element`s properties depend on the structure of its atoms
... become a dipole. • Now the two are more attracted to each other than they were before the induction occurred. • Ever induced behavior in another ...
... become a dipole. • Now the two are more attracted to each other than they were before the induction occurred. • Ever induced behavior in another ...
Nuclear Reactions Created by Patrick Haney The atoms of each
... Mass numbers do NOT tell us the number of neutrons in an isotope. To find the number of neutrons in an isotope, you must take the mass number and subtract the atomic number # of neutrons = mass number – atomic number How many neutrons are in the nucleus of a ...
... Mass numbers do NOT tell us the number of neutrons in an isotope. To find the number of neutrons in an isotope, you must take the mass number and subtract the atomic number # of neutrons = mass number – atomic number How many neutrons are in the nucleus of a ...
Dalton`s Atomic Theory
... COMPOUNDS are composed of the SAME TWO ELEMENTS then the RATIO of the masses of the SECOND ELEMENT combined with a certain mass of the FIRST ELEMENT is always a ratio of ...
... COMPOUNDS are composed of the SAME TWO ELEMENTS then the RATIO of the masses of the SECOND ELEMENT combined with a certain mass of the FIRST ELEMENT is always a ratio of ...
Chapter 2 Atoms, Molecules, and Ions
... 2.7 Ions and Ionic Compounds By gaining or losing one or more electrons neutral atoms become ions. • Cations (metals) are positive • Anions (nonmetals) are negative Ion Charge and the Periodic Table • The charge on an ion can often be determined from an element’s position on the Periodic Table • Met ...
... 2.7 Ions and Ionic Compounds By gaining or losing one or more electrons neutral atoms become ions. • Cations (metals) are positive • Anions (nonmetals) are negative Ion Charge and the Periodic Table • The charge on an ion can often be determined from an element’s position on the Periodic Table • Met ...
Chapter 4 Atomic Structure
... Based on his experimental evidence: The atom is mostly empty space All the positive charge, and almost all the mass is concentrated in a small area in the center. He called this a “nucleus” The nucleus is composed of protons and neutrons (they make the nucleus!) The electrons distributed aro ...
... Based on his experimental evidence: The atom is mostly empty space All the positive charge, and almost all the mass is concentrated in a small area in the center. He called this a “nucleus” The nucleus is composed of protons and neutrons (they make the nucleus!) The electrons distributed aro ...
2015 VCE Chemistry Unit 1 -Miss Fitzsimmons
... Every element in the first column (group one) has one electron in its outer shell. Every element on the second column (group two) has two electrons in the outer shell. As you keep counting the columns, you'll know how many electrons are in the outer shell. There are some exceptions to the order when ...
... Every element in the first column (group one) has one electron in its outer shell. Every element on the second column (group two) has two electrons in the outer shell. As you keep counting the columns, you'll know how many electrons are in the outer shell. There are some exceptions to the order when ...