study guide - atomic srtucture/_classification of matter
... idea that all things were made of particles too small to see. He was laughed at. In the 1800’s John Dalton proposed the idea of the “Atomic Theory”. He had 5 theories, 3 of which are still believed today. They are: 1. All matter is composed of extremely small particles too small to see 2. In reactio ...
... idea that all things were made of particles too small to see. He was laughed at. In the 1800’s John Dalton proposed the idea of the “Atomic Theory”. He had 5 theories, 3 of which are still believed today. They are: 1. All matter is composed of extremely small particles too small to see 2. In reactio ...
atoms
... foil which was only a few atoms thick. They found that although most of them passed through. About 1 in 10,000 hit. ...
... foil which was only a few atoms thick. They found that although most of them passed through. About 1 in 10,000 hit. ...
atoms - cloudfront.net
... arrangement of elements in which the elements are separated into groups based on a set of repeating properties The periodic table allows you to easily compare the properties of one element to another ...
... arrangement of elements in which the elements are separated into groups based on a set of repeating properties The periodic table allows you to easily compare the properties of one element to another ...
- Trinity Regional School
... the valence shell and is often unstable, meaning It does not contain the octet number of electrons In order for the atom to become stable or Fill the shell to the octet rule, this shell will be The one to bond. ...
... the valence shell and is often unstable, meaning It does not contain the octet number of electrons In order for the atom to become stable or Fill the shell to the octet rule, this shell will be The one to bond. ...
Chapter 3 pages 65
... of gold foil with alpha particles. He expected that the alpha particles would pass through the foil without being effected. When the experiment was over, he was surprised to see that some of the alpha particles actually bounced right back at him. He found that about 1 in 8000 did this. After thinkin ...
... of gold foil with alpha particles. He expected that the alpha particles would pass through the foil without being effected. When the experiment was over, he was surprised to see that some of the alpha particles actually bounced right back at him. He found that about 1 in 8000 did this. After thinkin ...
Atomic Theory PPT
... Each element consists of individual atoms All atoms of the same element are identical in mass and properties All atoms of different elements are different in mass and properties ...
... Each element consists of individual atoms All atoms of the same element are identical in mass and properties All atoms of different elements are different in mass and properties ...
Bio_130_files/Chemistry Review
... – Elements are the simplest form of matter with unique chemical properties. They are charted on the periodic table based on some of their chemical characteristics. • There are 24 major elements that have various roles in the body. – These include structural, enzymatic, and homeostatic balance. • Com ...
... – Elements are the simplest form of matter with unique chemical properties. They are charted on the periodic table based on some of their chemical characteristics. • There are 24 major elements that have various roles in the body. – These include structural, enzymatic, and homeostatic balance. • Com ...
1.2 Basic Atomic Theory Electrical structure of matter
... Atoms are not changed by chemical reactions Atoms cannot be created nor destroyed in chemical reactions (law of conservation of mass) Compounds form when elements combine ...
... Atoms are not changed by chemical reactions Atoms cannot be created nor destroyed in chemical reactions (law of conservation of mass) Compounds form when elements combine ...
Bio_130_files/Chemistry Review
... – Elements are the simplest form of matter with unique chemical properties. They are charted on the periodic table based on some of their chemical characteristics. • There are 24 major elements that have various roles in the body. – These include structural, enzymatic, and homeostatic balance. • Com ...
... – Elements are the simplest form of matter with unique chemical properties. They are charted on the periodic table based on some of their chemical characteristics. • There are 24 major elements that have various roles in the body. – These include structural, enzymatic, and homeostatic balance. • Com ...
Chapter 3.1 PPT
... the same proportions by mass regardless of the size of the sample or source of the compound • Law of multiple proportions: if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is ...
... the same proportions by mass regardless of the size of the sample or source of the compound • Law of multiple proportions: if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is ...
IntroRedoxDCIAns
... b. Identify two characteristics common to these equations. The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. ...
... b. Identify two characteristics common to these equations. The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. ...
Atomic Structure
... Definition : Isotopes are atoms of the same element with the same number of protons but different number of neutrons; Isotopes have the same atomic number but different mass numbers. ...
... Definition : Isotopes are atoms of the same element with the same number of protons but different number of neutrons; Isotopes have the same atomic number but different mass numbers. ...
Unit 3
... the same relative numbers and types of atoms. Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way atoms are grouped together. ...
... the same relative numbers and types of atoms. Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way atoms are grouped together. ...
2 - grade11chemistry
... • Putting all this together, we get B-R diagrams • To draw them you must know the # of protons, neutrons, and electrons (2,8,8,2 filling order) • Draw protons (p+), (n0) in circle (i.e. “nucleus”) • Draw electrons around in shells ...
... • Putting all this together, we get B-R diagrams • To draw them you must know the # of protons, neutrons, and electrons (2,8,8,2 filling order) • Draw protons (p+), (n0) in circle (i.e. “nucleus”) • Draw electrons around in shells ...
Joyce Wang
... challenged his view by saying there can be no indivisible particles • Because of their influence, the atomic view of matter faded for centuries • The Aristotelean philosophy dominated Western culture for centuries Aristotle ...
... challenged his view by saying there can be no indivisible particles • Because of their influence, the atomic view of matter faded for centuries • The Aristotelean philosophy dominated Western culture for centuries Aristotle ...
Atomic Structure
... Each ring of the Bohr Model is an energy level As you go further out from the nucleus the energy of ...
... Each ring of the Bohr Model is an energy level As you go further out from the nucleus the energy of ...
Exam Review - hrsbstaff.ednet.ns.ca
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
CHAPTER 4: ATOMS AND ELEMENTS
... – Group 2 or IIA: alkaline earth metals – Group 17 or VIIA: halogens – Group 18 or VIIIA: noble gases (because they are all gases that do not react) Transition Metals (or B Group Elements) – Elements in Groups 3 to 12 (middle of the Periodic Table) Inner Transition Elements (beneath the main body of ...
... – Group 2 or IIA: alkaline earth metals – Group 17 or VIIA: halogens – Group 18 or VIIIA: noble gases (because they are all gases that do not react) Transition Metals (or B Group Elements) – Elements in Groups 3 to 12 (middle of the Periodic Table) Inner Transition Elements (beneath the main body of ...
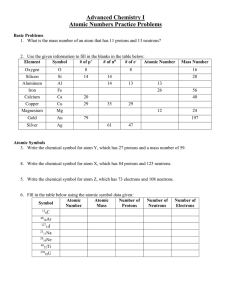
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Atoms - Chemistry Land
... Dalton is best known for his atomic theory, which revolutionized the science of chemistry and brought back Democritus’ concept of the atom. ...
... Dalton is best known for his atomic theory, which revolutionized the science of chemistry and brought back Democritus’ concept of the atom. ...
Models of the Atom and Periodic Trends Worksheet
... Group 14 elements have 4 valence electrons. Group 2 elements have 2 valence electrons . An electron dot formula of an elements shows the symbol of the element surrounded by its valence electrons. The ionization energy of an atom is the amount of energy required to remove an electron in the gaseous s ...
... Group 14 elements have 4 valence electrons. Group 2 elements have 2 valence electrons . An electron dot formula of an elements shows the symbol of the element surrounded by its valence electrons. The ionization energy of an atom is the amount of energy required to remove an electron in the gaseous s ...
Atomic Theory Notes
... • Electron-small mass, negative • Proton-much larger mass, positive • Neutron-about same mass as proton, no charge If this is your vision of an atom, you are back in the 1920’s!! ...
... • Electron-small mass, negative • Proton-much larger mass, positive • Neutron-about same mass as proton, no charge If this is your vision of an atom, you are back in the 1920’s!! ...