Bohr and Dot Diagrams Powerpoint
... Draw a circle to represent the nucleus of the atom. Write the number of protons and the number of neutrons inside this circle. Draw one or more circles around the nucleus depending on which row of the Periodic Table your element comes from. Each ring represents a different energy level for the elect ...
... Draw a circle to represent the nucleus of the atom. Write the number of protons and the number of neutrons inside this circle. Draw one or more circles around the nucleus depending on which row of the Periodic Table your element comes from. Each ring represents a different energy level for the elect ...
AtomicStructure_Peri..
... As you go across a period, the atoms gain protons while the number of electron energy levels remain the same. Positive protons and negative electrons attract each other. This increase in the number of protons causes a greater attraction between the nucleus and the electrons in the atom. As protons a ...
... As you go across a period, the atoms gain protons while the number of electron energy levels remain the same. Positive protons and negative electrons attract each other. This increase in the number of protons causes a greater attraction between the nucleus and the electrons in the atom. As protons a ...
по темі “Atoms and Molecules. The Periodic Table”
... 10.There is a progression from metals to non-metals across each period, isn’t there? 11.What does the block of elements in groups 3-12 contain? 12.Can the rare earth elements or transition metals be divided into lanthanides and actinides? ...
... 10.There is a progression from metals to non-metals across each period, isn’t there? 11.What does the block of elements in groups 3-12 contain? 12.Can the rare earth elements or transition metals be divided into lanthanides and actinides? ...
Bonding
... of atomic structure, explain wht these isotopes have in common, and how they differ. b.Write the complete electron configuration for a selenium atom in the ground state. Indicate the number of unpaired electrons in the ground-state atom, and explain your reasoning. ...
... of atomic structure, explain wht these isotopes have in common, and how they differ. b.Write the complete electron configuration for a selenium atom in the ground state. Indicate the number of unpaired electrons in the ground-state atom, and explain your reasoning. ...
Chapter 19: Molecules and Compounds
... Chapter 19: Molecules and Compounds Section 19.2 Chemical Formulas ...
... Chapter 19: Molecules and Compounds Section 19.2 Chemical Formulas ...
5 periodicity and atomic structure
... electrons. 3 Rules called the Aufbau (building up) principle guides the filling order of orbitals. The resultant lowest energy is called the ground- state electron configuration. Several orbitals will have the same energylevel which are said to be degenerate. ...
... electrons. 3 Rules called the Aufbau (building up) principle guides the filling order of orbitals. The resultant lowest energy is called the ground- state electron configuration. Several orbitals will have the same energylevel which are said to be degenerate. ...
Slide 1
... – Atomic number is the number of protons in an atom. – Mass number is the sum of protons and neutrons in an atom and indicates how much the atoms “weighs”—this is always a whole number. – Atomic mass (atomic weight) is the average mass of all naturally occurring isotopes—since this is an average, it ...
... – Atomic number is the number of protons in an atom. – Mass number is the sum of protons and neutrons in an atom and indicates how much the atoms “weighs”—this is always a whole number. – Atomic mass (atomic weight) is the average mass of all naturally occurring isotopes—since this is an average, it ...
File
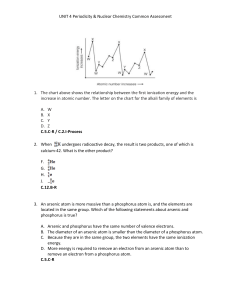
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
Atoms and Isotopes
... They are atoms of the same element that have different Number of Neutrons but must have the same number of Protons. ...
... They are atoms of the same element that have different Number of Neutrons but must have the same number of Protons. ...
Key - Seattle Central College
... water, fire, and earth. Aristotle (384-321 B.C.): accepted Empedocles idea and added a fifth element, heavenly ether, which is perfect, eternal, and incorruptible. Aristotle’s idea of five basic elements was accepted for 2000 years. John Dalton (1766-1844), an English chemist and physicist, establis ...
... water, fire, and earth. Aristotle (384-321 B.C.): accepted Empedocles idea and added a fifth element, heavenly ether, which is perfect, eternal, and incorruptible. Aristotle’s idea of five basic elements was accepted for 2000 years. John Dalton (1766-1844), an English chemist and physicist, establis ...
Bohr Atomic Model - Flinn Scientific
... energy of the appropriate wavelength. Conversely, an electron may “drop down” from a higher energy level to a lower energy level by releasing energy. The idea that only certain energy levels are allowed has proved enduring, and Niels Bohr was awarded the Nobel Prize in Physics in 1922 for his model ...
... energy of the appropriate wavelength. Conversely, an electron may “drop down” from a higher energy level to a lower energy level by releasing energy. The idea that only certain energy levels are allowed has proved enduring, and Niels Bohr was awarded the Nobel Prize in Physics in 1922 for his model ...
atomic theory of matter
... – The more cationlike element appears to the left of or below the other element in the periodic table ...
... – The more cationlike element appears to the left of or below the other element in the periodic table ...
Periodic Table Trends - Magoffin County Schools
... atoms are generally larger than group 18 atoms. • This is because, within a period, the number of principle energy levels (PELs) in each element generally remains constant. • For example, all elements in Period 3 have three energy levels. However, the nucleus gains protons as atomic number increases ...
... atoms are generally larger than group 18 atoms. • This is because, within a period, the number of principle energy levels (PELs) in each element generally remains constant. • For example, all elements in Period 3 have three energy levels. However, the nucleus gains protons as atomic number increases ...
Chapter 2. The Chemical Context of Life
... Elements & their valence shells Moving from left to right, each element has a sequential addition of electrons (and protons) ...
... Elements & their valence shells Moving from left to right, each element has a sequential addition of electrons (and protons) ...
How many electrons are present in a chromium
... If an element consists of 60.1% of atoms with a mass of 68.926 amu and the remainder of the atoms have a mass of 70.925 amu, what is the atomic mass of the element? Based on this mass, what do you think the identity of the element is? 100% - 60.1% = 39.9% of isotope 2 ...
... If an element consists of 60.1% of atoms with a mass of 68.926 amu and the remainder of the atoms have a mass of 70.925 amu, what is the atomic mass of the element? Based on this mass, what do you think the identity of the element is? 100% - 60.1% = 39.9% of isotope 2 ...
Mileposts on the road to the atom (download)
... Democritus posed the question: could matter be subdivided forever? He answered no: there is a limit to the extent to which matter can be subdivided, and he coined the term atom from the Greek for ...
... Democritus posed the question: could matter be subdivided forever? He answered no: there is a limit to the extent to which matter can be subdivided, and he coined the term atom from the Greek for ...
How many electrons are present in a chromium
... If an element consists of 60.1% of atoms with a mass of 68.926 amu and the remainder of the atoms have a mass of 70.925 amu, what is the atomic mass of the element? Based on this mass, what do you think the identity of the element is? 100% - 60.1% = 39.9% of isotope 2 ...
... If an element consists of 60.1% of atoms with a mass of 68.926 amu and the remainder of the atoms have a mass of 70.925 amu, what is the atomic mass of the element? Based on this mass, what do you think the identity of the element is? 100% - 60.1% = 39.9% of isotope 2 ...
File
... While working with cathode rays, Thomson discovered that they were made of tiny, identical and negatively charged particles which were later called ...
... While working with cathode rays, Thomson discovered that they were made of tiny, identical and negatively charged particles which were later called ...
Structure of the Atom JJ Thomson- discovered the electron in late
... as protons are found to be at the center of this nucleus. James Chadwick- discovers the NEUTRON in 1932. The neutron is located in the nucleus and has NO CHARGE. The following table summarizes the subatomic particles listed in order of discovery: ...
... as protons are found to be at the center of this nucleus. James Chadwick- discovers the NEUTRON in 1932. The neutron is located in the nucleus and has NO CHARGE. The following table summarizes the subatomic particles listed in order of discovery: ...
Atomic Structure
... ◦ all the positive charge and almost all the mass are concentrated in a small region that has enough positive charge to account for the great deflection of some of the alpha particles ◦ Nucleus: tiny, central core of an atom that is composed of neutrons and protons ◦ Electron are distributed around ...
... ◦ all the positive charge and almost all the mass are concentrated in a small region that has enough positive charge to account for the great deflection of some of the alpha particles ◦ Nucleus: tiny, central core of an atom that is composed of neutrons and protons ◦ Electron are distributed around ...
Avg. Atomic Mass - Greer Middle College
... He called the center of the atom the “____________” The nucleus is tiny compared to the atom as a whole. Rutherford reasoned that all of an atom’s positively charged particles were contained in the nucleus. The negatively charged particles were scattered outside the nucleus around the atom’s e ...
... He called the center of the atom the “____________” The nucleus is tiny compared to the atom as a whole. Rutherford reasoned that all of an atom’s positively charged particles were contained in the nucleus. The negatively charged particles were scattered outside the nucleus around the atom’s e ...