Questions - Scheikundeolympiade
... (case a) Pure aqueous solutions of HX were used. Only the undissociated species HX absorb. Curve A ( ), Curve B ( ), Curve C ( ), Curve D ( ) (case b) Pure aqueous solutions of HX were used. Only the anionic species X absorb. Curve A ( ), Curve B ( ), Curve C ( ), Curve D ( ) (case c) All solutions ...
... (case a) Pure aqueous solutions of HX were used. Only the undissociated species HX absorb. Curve A ( ), Curve B ( ), Curve C ( ), Curve D ( ) (case b) Pure aqueous solutions of HX were used. Only the anionic species X absorb. Curve A ( ), Curve B ( ), Curve C ( ), Curve D ( ) (case c) All solutions ...
pcc-sio2.alcohol.oxi..
... silica gel residues are then deposited in the solid waste containers for disposal.1 While the conversion of cis,trans-4-tertbutylcyclohexanol to the corresponding ketone gives superior results in terms of an undergraduate protocol, other secondary and primary alcohols (9) will serve as useful substr ...
... silica gel residues are then deposited in the solid waste containers for disposal.1 While the conversion of cis,trans-4-tertbutylcyclohexanol to the corresponding ketone gives superior results in terms of an undergraduate protocol, other secondary and primary alcohols (9) will serve as useful substr ...
First Year - WordPress.com
... they are isotopes compounds of both with CI2 will be similar in reactivity towards other compounds both have same colors both have same melting points ...
... they are isotopes compounds of both with CI2 will be similar in reactivity towards other compounds both have same colors both have same melting points ...
In Class Overview of Chapter
... When sufficient heat has been transferred to the liquid, another abrupt increase in entropy is observed. This occurs when the molecules have sufficient energy to overcome the intermolecular forces; this is when the liquid starts to boil. At greater temperatures, the substance exists as a gas and fur ...
... When sufficient heat has been transferred to the liquid, another abrupt increase in entropy is observed. This occurs when the molecules have sufficient energy to overcome the intermolecular forces; this is when the liquid starts to boil. At greater temperatures, the substance exists as a gas and fur ...
p Block Elements General Configuration: ns2 np1
... electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-pπ bonds as their atomic orbitals are so large and differs that they cannot have effective ...
... electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-pπ bonds as their atomic orbitals are so large and differs that they cannot have effective ...
9182747 Chemistry Ja02
... (1) They are determined by the number of neutrons. (2) They are determined by the number of electrons in the first shell. (3) They change in a generally systematic ...
... (1) They are determined by the number of neutrons. (2) They are determined by the number of electrons in the first shell. (3) They change in a generally systematic ...
введение в общую introductio to the general ch ведение в общую
... The question from the title of this subsection is very important. It can be rephrased in the following ways. What is chemistry? What is the subject of the discipline you are starting (or, hopefully, continuing) to study? What is the difference between chemistry and physics? Physical properties of a ...
... The question from the title of this subsection is very important. It can be rephrased in the following ways. What is chemistry? What is the subject of the discipline you are starting (or, hopefully, continuing) to study? What is the difference between chemistry and physics? Physical properties of a ...
Chapter 3: Calculations with Chemical Formulas
... The alkaline earth metals (Be, Mg, Ca, Sr, Ba, and Ra) and also Zn and Cd in compounds are always assigned an oxidation state of +2. Hydrogen in compounds is assigned an oxidation state of +1. Oxygen in compounds is assigned an oxidation state of -2. Halogen in compounds is assigned an oxidation sta ...
... The alkaline earth metals (Be, Mg, Ca, Sr, Ba, and Ra) and also Zn and Cd in compounds are always assigned an oxidation state of +2. Hydrogen in compounds is assigned an oxidation state of +1. Oxygen in compounds is assigned an oxidation state of -2. Halogen in compounds is assigned an oxidation sta ...
Chemistry Final Exam Review
... • basic characteristics and names of the major groups • metals, nonmetals, metalloids – “staircase” • ionization energy, electronegativity, atomic radius, trends shown in these properties on the periodic table Problems: 1. Give the number of valence electrons, physical state (metal, nonmetal, or met ...
... • basic characteristics and names of the major groups • metals, nonmetals, metalloids – “staircase” • ionization energy, electronegativity, atomic radius, trends shown in these properties on the periodic table Problems: 1. Give the number of valence electrons, physical state (metal, nonmetal, or met ...
Organic Chemistry 2014 finalzzz
... Number the carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (use the suffix –yl for branches) Write the complete IUPAC name, following the format: (number of ...
... Number the carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (use the suffix –yl for branches) Write the complete IUPAC name, following the format: (number of ...
XIX. Chemistry, High School
... Each student taking the high school Chemistry test was provided with a Chemistry Formula and Constants Sheet/Periodic Table of the Elements. Copies of both sides of this formula sheet follow the final question in this chapter. Each student also had sole access to a calculator with at least four func ...
... Each student taking the high school Chemistry test was provided with a Chemistry Formula and Constants Sheet/Periodic Table of the Elements. Copies of both sides of this formula sheet follow the final question in this chapter. Each student also had sole access to a calculator with at least four func ...
Exercise #5_Chpt 2
... 2. Sulfur and oxygen can react to form both sulfur dioxide and sulfur trioxide . In sulfur dioxide, 32.06 g of sulfur are combined with 32.00 g of oxygen. In sulfur trioxide, 32.06 g of sulfur are combined with 48.00 g a. What is the ratio of the masses of oxygen that combine with 32.06 g of sulfur? ...
... 2. Sulfur and oxygen can react to form both sulfur dioxide and sulfur trioxide . In sulfur dioxide, 32.06 g of sulfur are combined with 32.00 g of oxygen. In sulfur trioxide, 32.06 g of sulfur are combined with 48.00 g a. What is the ratio of the masses of oxygen that combine with 32.06 g of sulfur? ...
chem 13 news 2010 - University of Waterloo
... STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect ...
... STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect ...
Final Exam Review Packet
... ____ 45. Sulfuric acid is probably the most important industrial chemical because it is used in so many industrial processes to produce or purify other chemicals. It can be produced by a three step process. First, sulfur is burned in air to give sulfur dioxide. Second, the sulfur dioxide is converte ...
... ____ 45. Sulfuric acid is probably the most important industrial chemical because it is used in so many industrial processes to produce or purify other chemicals. It can be produced by a three step process. First, sulfur is burned in air to give sulfur dioxide. Second, the sulfur dioxide is converte ...
Name AP Chemistry Take Home Quiz – Due Thursday, 1/9/2014
... a. CN-(aq) is a stronger base than C2H3O2-(aq) b. HCN(aq) is a stronger acid than HC2H3O2(aq) c. The conjugate base of CN-(aq) is C2H3O2-(aq) d. The equilibrium constant will increase with an increase in temperature. e. The pH of a solution containing equimolar amounts of CN-(aq) and HC2H3O2(aq) is ...
... a. CN-(aq) is a stronger base than C2H3O2-(aq) b. HCN(aq) is a stronger acid than HC2H3O2(aq) c. The conjugate base of CN-(aq) is C2H3O2-(aq) d. The equilibrium constant will increase with an increase in temperature. e. The pH of a solution containing equimolar amounts of CN-(aq) and HC2H3O2(aq) is ...
Stoichiometry - HCC Learning Web
... Step 3: Calculate the moles of "desired" substance from your answer in Step 2 using the coefficients from the balanced chemical equation. If more than one reactant was given originally, you can calculate the moles of product twice, based on the moles of each reactant. The reactant that gives the sma ...
... Step 3: Calculate the moles of "desired" substance from your answer in Step 2 using the coefficients from the balanced chemical equation. If more than one reactant was given originally, you can calculate the moles of product twice, based on the moles of each reactant. The reactant that gives the sma ...
LECTURE_Solutions2013(1)
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
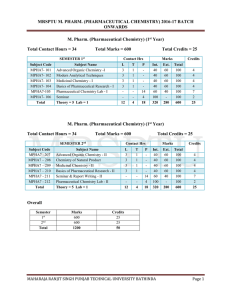
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Infrared Spectroscopy: The Hook’s Law and Calculation of Stretching Frequencies for Different Types of Bonds And Their Bond Strengths, Coupled Interactions, Hydrogen Bonding, Examination of Infrared Spectrum, Survey of Important Functional Groups With Examples, Radiation Source, Detectors Used, Samp ...
... Infrared Spectroscopy: The Hook’s Law and Calculation of Stretching Frequencies for Different Types of Bonds And Their Bond Strengths, Coupled Interactions, Hydrogen Bonding, Examination of Infrared Spectrum, Survey of Important Functional Groups With Examples, Radiation Source, Detectors Used, Samp ...
Slide 1
... Predicting the Stability of Aqueous Ions The relative stability of Cr and Cr2+ can be explain as follow: Cr2+ + 2e Cr EӨ = -0.91 V 2H+ + 2e H2 EӨ = 0.00 V ------------------------------------------------------Cr + 2H+ Cr2+ + H2 EӨ = + 0.91V ...
... Predicting the Stability of Aqueous Ions The relative stability of Cr and Cr2+ can be explain as follow: Cr2+ + 2e Cr EӨ = -0.91 V 2H+ + 2e H2 EӨ = 0.00 V ------------------------------------------------------Cr + 2H+ Cr2+ + H2 EӨ = + 0.91V ...
File - chemistryattweed
... The oxides of elements have increasingly acidic character going from left to right across a period. Sodium and magnesium oxides are bases. Aluminium oxide is amphoteric, (it shows both acidic and basic characteristics). The oxides of silicon, phosphorus, sulfur and chlorine are all acidic but vary i ...
... The oxides of elements have increasingly acidic character going from left to right across a period. Sodium and magnesium oxides are bases. Aluminium oxide is amphoteric, (it shows both acidic and basic characteristics). The oxides of silicon, phosphorus, sulfur and chlorine are all acidic but vary i ...
Fall 2002 Honors
... 15. (5 pts) Nitrogen and phosphorus are in the same group, so you would expect them to exhibit similar chemical properties. NCl3, PCl3, and PCl5 are all stable compounds that are easily synthesized in the lab. However, NCl5 has never been synthesized or observed. Why would phosphorus form two compou ...
... 15. (5 pts) Nitrogen and phosphorus are in the same group, so you would expect them to exhibit similar chemical properties. NCl3, PCl3, and PCl5 are all stable compounds that are easily synthesized in the lab. However, NCl5 has never been synthesized or observed. Why would phosphorus form two compou ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.