AP Chemistry - Oak Park Unified School District
... being at a particular point in space is given by its (3). An orbital is described by a combination of four quantum numbers. The principal quantum number, n, is indicated by the integers 1, 2, 3. . . 7. This quantum number relates to the (4) and energy of the orbital. The sublevel quantum number, l, ...
... being at a particular point in space is given by its (3). An orbital is described by a combination of four quantum numbers. The principal quantum number, n, is indicated by the integers 1, 2, 3. . . 7. This quantum number relates to the (4) and energy of the orbital. The sublevel quantum number, l, ...
Chapter 5
... H2SO4 are needed to react completely with 25.0 mL of 0.400 M NaOH? 2 NaOH(aq) + H2SO4(aq) -----> Na2SO4(aq) + 2 H2O (25.0 mL NaOH) (0.400 mol NaOH) (1 L) (1 mol H2SO4) --------------------------------#mL H2SO4 = ----------------------------------------------(1 L NaOH) (1000 mL)(2 mol NaOH) ...
... H2SO4 are needed to react completely with 25.0 mL of 0.400 M NaOH? 2 NaOH(aq) + H2SO4(aq) -----> Na2SO4(aq) + 2 H2O (25.0 mL NaOH) (0.400 mol NaOH) (1 L) (1 mol H2SO4) --------------------------------#mL H2SO4 = ----------------------------------------------(1 L NaOH) (1000 mL)(2 mol NaOH) ...
Electrochemical Wet Etching of Silver STM Tips
... • Prevent Solution concentration for changing – Retard electrolyte evaporation ...
... • Prevent Solution concentration for changing – Retard electrolyte evaporation ...
Chapter 1 Introduction
... sulphides of metals such as lead and zinc and of course gemstones such as ruby (Al2O3) and diamond (C). For many centuries the word ―crystal‖ was applied specifically to quartz, it is based on the Greek word implying a form similar to that of ice. In current usage, a crystalline solid is one in whic ...
... sulphides of metals such as lead and zinc and of course gemstones such as ruby (Al2O3) and diamond (C). For many centuries the word ―crystal‖ was applied specifically to quartz, it is based on the Greek word implying a form similar to that of ice. In current usage, a crystalline solid is one in whic ...
File
... Average Bond Enthalpy is the average enthalpy change when breaking 1 mole of bonds in a compound which is in its gaseous state. Breaking Bonds is an endothermic process. It has a positive sign, as energy must be put into the system to break the chemical bonds. Making bonds in an exothermic process. ...
... Average Bond Enthalpy is the average enthalpy change when breaking 1 mole of bonds in a compound which is in its gaseous state. Breaking Bonds is an endothermic process. It has a positive sign, as energy must be put into the system to break the chemical bonds. Making bonds in an exothermic process. ...
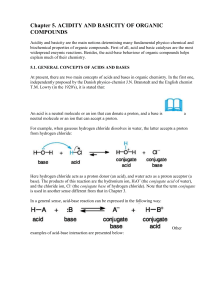
Chapter 5. ACIDITY AND BASICITY OF ORGANIC COMPOUNDS
... Another stabilizing factor is the polarizability (opposed to polarity) of an element in the acidic site. This term means the ability of the electrons to respond to a changing electric field, as a result of its interaction with solvent or with other polar reagents. Relative polarizability increases ...
... Another stabilizing factor is the polarizability (opposed to polarity) of an element in the acidic site. This term means the ability of the electrons to respond to a changing electric field, as a result of its interaction with solvent or with other polar reagents. Relative polarizability increases ...
enthalpy 2
... is the standard enthalpy of formation is the enthalpy change when 1 mole of compound is produced from its elements in standard conditions, all products and reactants in the standard state. The standard state is important, it means the way that the element is, at standard conditions (see above). So y ...
... is the standard enthalpy of formation is the enthalpy change when 1 mole of compound is produced from its elements in standard conditions, all products and reactants in the standard state. The standard state is important, it means the way that the element is, at standard conditions (see above). So y ...
Analyze
... Octane at –57˚C is a solid just about to melt. As energy is added the solid octane melts and its temperature does not change until all the solid is melted. Only when octane is entirely liquid does added energy increase the temperature of the liquid until the boiling point of octane is reached. Durin ...
... Octane at –57˚C is a solid just about to melt. As energy is added the solid octane melts and its temperature does not change until all the solid is melted. Only when octane is entirely liquid does added energy increase the temperature of the liquid until the boiling point of octane is reached. Durin ...
Gas-phase study of the reactivity of optical coating desktop-size extreme-ultraviolet laser
... equipment is given below. MmOn (M = Si, Ti, Hf, or Zr) clusters are generated by laser ablation with a focused 532 nm laser (Nd3+ : YAG, 10 Hz, 5 – 8 mJ/ cm2, 8 ns duration) onto a 12 mm diameter spring-loaded metal disk in the presence of a pulsed helium carrier gas mixed with 5% O2, controlled by ...
... equipment is given below. MmOn (M = Si, Ti, Hf, or Zr) clusters are generated by laser ablation with a focused 532 nm laser (Nd3+ : YAG, 10 Hz, 5 – 8 mJ/ cm2, 8 ns duration) onto a 12 mm diameter spring-loaded metal disk in the presence of a pulsed helium carrier gas mixed with 5% O2, controlled by ...
Chemistry English
... Atomic theory: if the matter were divided a sufficient number of times, it could eventually be reduced to the indivisible, indestructible particles called atom. The atomic theory was presented by the British chemist John Dalton (1766-1844) in the early 1800s. It is one of the greatest advances in th ...
... Atomic theory: if the matter were divided a sufficient number of times, it could eventually be reduced to the indivisible, indestructible particles called atom. The atomic theory was presented by the British chemist John Dalton (1766-1844) in the early 1800s. It is one of the greatest advances in th ...
- Vijay Education Academy
... 37. Maneesh, a student of class XII, watched a programme on TV where it was being shown how use of polythene bags blocked the sewer system and how sometimes the polythene bags thrown as garbage into the streets were swallowed by the animals resulting into their death. Maneesh was highly upset and he ...
... 37. Maneesh, a student of class XII, watched a programme on TV where it was being shown how use of polythene bags blocked the sewer system and how sometimes the polythene bags thrown as garbage into the streets were swallowed by the animals resulting into their death. Maneesh was highly upset and he ...
Stoichiometry intro
... reaction represent the ratio of the moles of substances that react and form during a chemical reaction. These numbers are fixed - they do not change We can use these ratios to predict the amounts of substances that react and form in a reaction when any amount of a substance is reacted. The conve ...
... reaction represent the ratio of the moles of substances that react and form during a chemical reaction. These numbers are fixed - they do not change We can use these ratios to predict the amounts of substances that react and form in a reaction when any amount of a substance is reacted. The conve ...
chemistry (9189)
... industrial and laboratory visits relevant to the content of the options chosen. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, learning Outcomes have been used throughout. Each part of the syllabus is specified by a Content ...
... industrial and laboratory visits relevant to the content of the options chosen. In order to specify the syllabus as precisely as possible and also to emphasise the importance of skills other than recall, learning Outcomes have been used throughout. Each part of the syllabus is specified by a Content ...
Dielectric and thermodynamic response of a
... collection of charges in a spherical cavity which is immersed in a continuum dielectric medium. In this model, the reaction field potential arising from the charges in the spherical cavity can be expressed as an infinite series, each term of which is the reaction field arising from a corresponding m ...
... collection of charges in a spherical cavity which is immersed in a continuum dielectric medium. In this model, the reaction field potential arising from the charges in the spherical cavity can be expressed as an infinite series, each term of which is the reaction field arising from a corresponding m ...
THERMODYNAMICS OF REACTING SYSTEMS
... For example, if in combustion of carbon, there is also carbon monoxide present, then two reactions are needed to describe the system. They can be as given below: 2C ...
... For example, if in combustion of carbon, there is also carbon monoxide present, then two reactions are needed to describe the system. They can be as given below: 2C ...
Density Functional Theory Based Study of the Electron Transfer
... relatively wide electrochemical stability window and negligible vapor pressure, are promising candidates as electrolytes for developing lithium−air batteries with enhanced performance. The local current density, a crucial parameter in determining the performance of lithium−air batteries, is directly ...
... relatively wide electrochemical stability window and negligible vapor pressure, are promising candidates as electrolytes for developing lithium−air batteries with enhanced performance. The local current density, a crucial parameter in determining the performance of lithium−air batteries, is directly ...
Chemical and physical changes
... however, even though a current electrical passes through them or they are warmed up at very high temperatures, these substances continue being the same ones. Susana Morales Bernal ...
... however, even though a current electrical passes through them or they are warmed up at very high temperatures, these substances continue being the same ones. Susana Morales Bernal ...
Balanced Equations And Equilibrium Constants
... Given overall reaction (need to REVERSE first reaction, see Rule 2 for how this changes the equilibrium constant): N2O(g) + (1/2)O2(g) <---> 2NO(g) K = (1/K1)*(K2) = 8.5*10-13 2) When we reverse an equation we invert the value of K. Kreverse reaction = 1/(Kforward reaction) 3) When we multiply coeff ...
... Given overall reaction (need to REVERSE first reaction, see Rule 2 for how this changes the equilibrium constant): N2O(g) + (1/2)O2(g) <---> 2NO(g) K = (1/K1)*(K2) = 8.5*10-13 2) When we reverse an equation we invert the value of K. Kreverse reaction = 1/(Kforward reaction) 3) When we multiply coeff ...
45. kinetics ch 12
... The physical state of the reactants: The ability of the reactants to collide lead to a reaction. The more readily molecules collide with each other, the more rapidly they react. A solid that is broken in up in to pieces will react faster than one that is not because of a greater surface area for rea ...
... The physical state of the reactants: The ability of the reactants to collide lead to a reaction. The more readily molecules collide with each other, the more rapidly they react. A solid that is broken in up in to pieces will react faster than one that is not because of a greater surface area for rea ...
National 5 - Deans Community High School
... 23. Most polymers are insoluble in water. Some hospitals collect dirty clothing and bedding in bags made from a special polymer which dissolves in hot water. These bags can be sealed and put into washing machines. The risk of infection from touching dirty material is less. You have been given severa ...
... 23. Most polymers are insoluble in water. Some hospitals collect dirty clothing and bedding in bags made from a special polymer which dissolves in hot water. These bags can be sealed and put into washing machines. The risk of infection from touching dirty material is less. You have been given severa ...
the powerpoint
... elements composing the substances. • In a chemical formula, the numbers as subscripts show how many of each kind of atom are in the compound. • The subscript is written to the lower right of the element symbol. • If no subscript is written, only one atom of that element is part of the compound. For ...
... elements composing the substances. • In a chemical formula, the numbers as subscripts show how many of each kind of atom are in the compound. • The subscript is written to the lower right of the element symbol. • If no subscript is written, only one atom of that element is part of the compound. For ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.