Synthesis of Imidazolium Room-Temperature Ionic
... to remove colored impurities either at the stage of the bromide or later (10), if spectroscopic grade ionic liquids are desired. Carrying out the reaction in a solvent ensures that the resulting ionic liquid does not develop a brown color. The one-pot procedure is versatile and can be done in a vari ...
... to remove colored impurities either at the stage of the bromide or later (10), if spectroscopic grade ionic liquids are desired. Carrying out the reaction in a solvent ensures that the resulting ionic liquid does not develop a brown color. The one-pot procedure is versatile and can be done in a vari ...
chm 205 - National Open University of Nigeria
... allotropes, and the phenomenon is called allotropy. The two common allotropic forms of carbon, viz., diamond and graphite are well-known. These are, in fact, giant macromolecules consisting of C atoms linked by a network of covalent bonds (Figs, 1.l and 1.2). Each carbon in diamond is tetrahedrally ...
... allotropes, and the phenomenon is called allotropy. The two common allotropic forms of carbon, viz., diamond and graphite are well-known. These are, in fact, giant macromolecules consisting of C atoms linked by a network of covalent bonds (Figs, 1.l and 1.2). Each carbon in diamond is tetrahedrally ...
CHAPTER 4 - Myschoolpages.com
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
Now! - Soojeede.com
... an accomplishment for a scientist in the late 19 century with very little technology but with the combination of knowledge and intellect available at the time. Arrhenius led the way to our understanding of how acids and bases differed, their properties, and their reactions. We may not realize how mu ...
... an accomplishment for a scientist in the late 19 century with very little technology but with the combination of knowledge and intellect available at the time. Arrhenius led the way to our understanding of how acids and bases differed, their properties, and their reactions. We may not realize how mu ...
2014 International Practice Exam: Chemistry
... Are there any questions? . . . Section I is the multiple-choice portion of the exam. You may never discuss these specific multiple-choice questions at any time in any form with anyone, including your teacher and other students. If you disclose these questions through any means, your AP Exam score wi ...
... Are there any questions? . . . Section I is the multiple-choice portion of the exam. You may never discuss these specific multiple-choice questions at any time in any form with anyone, including your teacher and other students. If you disclose these questions through any means, your AP Exam score wi ...
Homework1-4-Answers
... 22. Phosphorus reacts with iodine as shown in the chemical reaction below. What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of ...
... 22. Phosphorus reacts with iodine as shown in the chemical reaction below. What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of ...
First Law of Thermodynamics
... In our study of thermodynamics, we will be interested in the changes in the thermodynamic properties that accompany a process. A change is designated by the symbol “Δ”. For example, “ΔX” means a change in the quantity X. The sign of ΔX indicates the direction of the change because ΔX ≡ Xfinal - Xini ...
... In our study of thermodynamics, we will be interested in the changes in the thermodynamic properties that accompany a process. A change is designated by the symbol “Δ”. For example, “ΔX” means a change in the quantity X. The sign of ΔX indicates the direction of the change because ΔX ≡ Xfinal - Xini ...
$doc.title
... For the path A → C → B , work is done by the field only along the segment AC that is parallel to the field lines. Points B and C are at the same electric potential, i.e., VB = VC . Because ΔU = qΔV , this means that no work is required when moving the charge from B to C. In fact, all points along th ...
... For the path A → C → B , work is done by the field only along the segment AC that is parallel to the field lines. Points B and C are at the same electric potential, i.e., VB = VC . Because ΔU = qΔV , this means that no work is required when moving the charge from B to C. In fact, all points along th ...
Chapter 6 Table of Contents
... Note how the mol H2 unit cancels, and mol O2 is the new unit introduced. This is an example of a mole-mole calculation, when you start with moles of one substance and convert to moles of another substance by using the balanced chemical equation. The example may seem simple because the numbers are sm ...
... Note how the mol H2 unit cancels, and mol O2 is the new unit introduced. This is an example of a mole-mole calculation, when you start with moles of one substance and convert to moles of another substance by using the balanced chemical equation. The example may seem simple because the numbers are sm ...
Slides
... ! Associated with breaking bonds or Intermolecular forces. ! Heat energy goes into a system to break the bonds or pull ...
... ! Associated with breaking bonds or Intermolecular forces. ! Heat energy goes into a system to break the bonds or pull ...
HSC Chemistry Syllabus Notes 2007
... The Prescribed Focus Areas are different curriculum emphases or purposes designed to increase students’ understanding of chemistry as an ever-developing body of knowledge, the provisional nature of scientific explanations in chemistry, the complex relationship between evidence and ideas in chemistry ...
... The Prescribed Focus Areas are different curriculum emphases or purposes designed to increase students’ understanding of chemistry as an ever-developing body of knowledge, the provisional nature of scientific explanations in chemistry, the complex relationship between evidence and ideas in chemistry ...
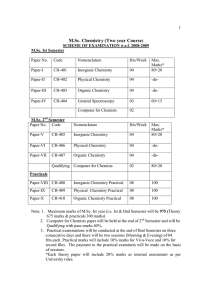
M.Sc. Chemistry (Two year Course)
... Entropy changes in reversible and irreversible processes; variation of entropy with temperature , pressure and volume, entropy concept as a measure of unavailable energy and criteria for the spontaneity of reaction; free energy functions and their significance, criteria for spontaneity of a process; ...
... Entropy changes in reversible and irreversible processes; variation of entropy with temperature , pressure and volume, entropy concept as a measure of unavailable energy and criteria for the spontaneity of reaction; free energy functions and their significance, criteria for spontaneity of a process; ...
Introduction to Chemistry
... 2. I can calculate the pH of a solution. 3. I can write a neutralization reaction between an acid and base. 4. I can calculate the concentration of an acid or base from data collected in a titration. Unit 9: Energy of Chemical Changes Nature of Science Goal—Science provides technology to improve liv ...
... 2. I can calculate the pH of a solution. 3. I can write a neutralization reaction between an acid and base. 4. I can calculate the concentration of an acid or base from data collected in a titration. Unit 9: Energy of Chemical Changes Nature of Science Goal—Science provides technology to improve liv ...
PDF w - ACS Publications - American Chemical Society
... Continuous Photolysis and KIE Measurements. Continuous photolysis was carried out in NMR tubes sealed with J. Young valves, and reaction progress was monitored by 1H NMR spectroscopy. NMR spectra were obtained on a Bruker Avance III 400 FT NMR spectrometer (400 MHz for 1H). Solutions of [(η5-iPr4C5H ...
... Continuous Photolysis and KIE Measurements. Continuous photolysis was carried out in NMR tubes sealed with J. Young valves, and reaction progress was monitored by 1H NMR spectroscopy. NMR spectra were obtained on a Bruker Avance III 400 FT NMR spectrometer (400 MHz for 1H). Solutions of [(η5-iPr4C5H ...
Review Packet Answers - Bremerton School District
... The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. That decrease realized by the formation of more solid NH4HS. Kp = (PNH3) (PH2S) (A complete ...
... The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. That decrease realized by the formation of more solid NH4HS. Kp = (PNH3) (PH2S) (A complete ...
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
base hydrolysis of cobalt(iii)
... I was pleased to be invited by Professor D. A. Davenport to present a paper at the symposium C. K. Ingold: Master and Mandarin of Physical Organic Chemistry to honor Professor Sir Christopher Ingold on the centennial year of his birth. The chemistry community recalls that he was one of the giants of ...
... I was pleased to be invited by Professor D. A. Davenport to present a paper at the symposium C. K. Ingold: Master and Mandarin of Physical Organic Chemistry to honor Professor Sir Christopher Ingold on the centennial year of his birth. The chemistry community recalls that he was one of the giants of ...
Synthesis and Characterisation of N
... arrangement forced by the bridging methylene. Oxidation and metal coordination reactions are performed on such compounds. ...
... arrangement forced by the bridging methylene. Oxidation and metal coordination reactions are performed on such compounds. ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.