The interaction between colloids in polar mixtures above Tc
... The dashed and dashed-dotted curves in Fig. 1(a) are the same as the solid curve for τ = 0.008 except for one parameter. The effect of charge asymmetry is shown by the dasheddotted curve, where the total charge was kept constant, but σ L = 3σ R . Here the attraction is stronger, as in the classic Po ...
... The dashed and dashed-dotted curves in Fig. 1(a) are the same as the solid curve for τ = 0.008 except for one parameter. The effect of charge asymmetry is shown by the dasheddotted curve, where the total charge was kept constant, but σ L = 3σ R . Here the attraction is stronger, as in the classic Po ...
CHAPTER 19 TRANSITION METALS AND COORDINATION
... electrons that can be removed. Stable ions of the representative metals are determined by how many s and p valence electrons can be removed. In general, representative metals lose all of the s and p valence electrons to form their stable ions. Transition metals generally lose the s electron(s) to fo ...
... electrons that can be removed. Stable ions of the representative metals are determined by how many s and p valence electrons can be removed. In general, representative metals lose all of the s and p valence electrons to form their stable ions. Transition metals generally lose the s electron(s) to fo ...
Chlorine Chemistry For Water and Waste Treatment
... NOTE: Methods are available for both laboratory and continuous measurement of Free Chlorine Residual and Total Chlorine Residual. Combined Chlorine Residual must be determined by subtracting Free residual from Total. Available Cl2 - The term “available “chlorine is commonly used. It means, simply, t ...
... NOTE: Methods are available for both laboratory and continuous measurement of Free Chlorine Residual and Total Chlorine Residual. Combined Chlorine Residual must be determined by subtracting Free residual from Total. Available Cl2 - The term “available “chlorine is commonly used. It means, simply, t ...
Recycling of the used cooking oils as corrosion inhibitors
... where, Kads is equilibrium constant of the equilibrium adsorption process. This isotherm assumes that adsorbed molecule occupies only one site and it does not interact with other adsorbed species. The Kads values can be calculated from the intercept lines on the Cinhi/ Ѳ axis. This is related to the ...
... where, Kads is equilibrium constant of the equilibrium adsorption process. This isotherm assumes that adsorbed molecule occupies only one site and it does not interact with other adsorbed species. The Kads values can be calculated from the intercept lines on the Cinhi/ Ѳ axis. This is related to the ...
Equilibrium - Clayton State University
... - Many reactions do not go to completion - Amount of products formed or reactants consumed cannot be predicted from stoichiometry alone - These reactions achieve a condition of equilibrium ...
... - Many reactions do not go to completion - Amount of products formed or reactants consumed cannot be predicted from stoichiometry alone - These reactions achieve a condition of equilibrium ...
PDF - mockies – Mockiesgateacademy
... In using the term mole for ionic substances, we mean the number of formula units of the substance. For example, a mole of sodium carbonate, Na2CO3 is a quantity containing 6.023 x 1023 Na2CO3 units. But each formula unit of Na2CO3 contains 2 x 6.023 x 1023 Na+ ions and one CO32ions and 1 x 6.023 x 1 ...
... In using the term mole for ionic substances, we mean the number of formula units of the substance. For example, a mole of sodium carbonate, Na2CO3 is a quantity containing 6.023 x 1023 Na2CO3 units. But each formula unit of Na2CO3 contains 2 x 6.023 x 1023 Na+ ions and one CO32ions and 1 x 6.023 x 1 ...
+2 - h2ochem
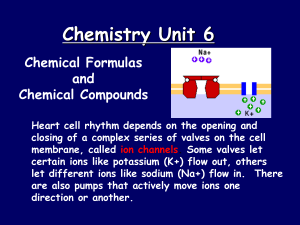
... closing of a complex series of valves on the cell membrane, called ion channels. Some valves let certain ions like potassium (K+) flow out, others let different ions like sodium (Na+) flow in. There are also pumps that actively move ions one direction or another. ...
... closing of a complex series of valves on the cell membrane, called ion channels. Some valves let certain ions like potassium (K+) flow out, others let different ions like sodium (Na+) flow in. There are also pumps that actively move ions one direction or another. ...
1.8 M - Thierry Karsenti
... the first half of the basic first year course i.e., General chemistry, module 1, we examined the concepts that underpin matter and measurement, atomic structure and periodicity. In this module we will look more closely at chemical reactions and the energy laws that govern them. Most chemical reactio ...
... the first half of the basic first year course i.e., General chemistry, module 1, we examined the concepts that underpin matter and measurement, atomic structure and periodicity. In this module we will look more closely at chemical reactions and the energy laws that govern them. Most chemical reactio ...
Chemistry Curriculum Map - Belle Vernon Area School District
... radioactive decay and compare their properties. Standard: 3.2.C.A3 – Describe the process of radioactive decay by using nuclear equations and explain the concept of half-life for an isotope. ...
... radioactive decay and compare their properties. Standard: 3.2.C.A3 – Describe the process of radioactive decay by using nuclear equations and explain the concept of half-life for an isotope. ...
Chemistry Challenge Problems
... was made by the German chemist Johann Wolfgang Döbereiner (1780–1849). In 1816, Döbereiner noticed that the then accepted atomic mass of strontium (50) was midway between the atomic masses of calcium (27.5) and barium (72.5). Note that the accepted atomic masses for these elements today are very dif ...
... was made by the German chemist Johann Wolfgang Döbereiner (1780–1849). In 1816, Döbereiner noticed that the then accepted atomic mass of strontium (50) was midway between the atomic masses of calcium (27.5) and barium (72.5). Note that the accepted atomic masses for these elements today are very dif ...
Organic Chemistry II Introduction
... Enolization • conditions permit the enolization of ketones • Substitution can occur at the alpha position through either an enol or enolate ion ...
... Enolization • conditions permit the enolization of ketones • Substitution can occur at the alpha position through either an enol or enolate ion ...
Reduction of CuO in H2: in situ time
... In our experiments with pure powders of CuO, we found reaction with H2 and reduction to metallic copper at temperatures between 150 and 300 8C. A similar fact has been found in H2 -TPR experiments for the reduction of CuO supported on Al2 O3 [18] or mixed with ZnO [13]. Figure 1 shows time-resolved ...
... In our experiments with pure powders of CuO, we found reaction with H2 and reduction to metallic copper at temperatures between 150 and 300 8C. A similar fact has been found in H2 -TPR experiments for the reduction of CuO supported on Al2 O3 [18] or mixed with ZnO [13]. Figure 1 shows time-resolved ...
entropy - KFUPM Faculty List
... be spontaneous as written (in the for- ward direction), Suniv must be positive. An equilibrium process is one that does not occur spontaneously in either the net forward or net reverse direction but can be made to occur by the addition or removal of energy to a system at equilibrium. ...
... be spontaneous as written (in the for- ward direction), Suniv must be positive. An equilibrium process is one that does not occur spontaneously in either the net forward or net reverse direction but can be made to occur by the addition or removal of energy to a system at equilibrium. ...
B.Sc. (CHEMISTRY) - Dr B. R. Ambedkar University
... inversion, retention and recemization. Relative and absolute configuration, sequence rules, D & L and R & S systems of nomenclature. Geometric isomerism – Determination of configuration of geometric isomers. E & Z system of nomenclature, geometric isomerism in oximes and ...
... inversion, retention and recemization. Relative and absolute configuration, sequence rules, D & L and R & S systems of nomenclature. Geometric isomerism – Determination of configuration of geometric isomers. E & Z system of nomenclature, geometric isomerism in oximes and ...
Table of Contents - slccscience`s Home Page
... elements, it often seems odd that an entire branch of chemistry is devoted to a single element and its compounds while the other 116 elements and their compounds are all lumped together in a separate discipline, but there is a very good reason for this. There are about 1.5 million known inorganic co ...
... elements, it often seems odd that an entire branch of chemistry is devoted to a single element and its compounds while the other 116 elements and their compounds are all lumped together in a separate discipline, but there is a very good reason for this. There are about 1.5 million known inorganic co ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.