CHE 1031 Lab Manual
... Knowledge in chemistry, as in all the physical sciences, is obtained initially from performing experiments in a laboratory. It is in the laboratory that facts are discovered and concepts, ideas and theorie ...
... Knowledge in chemistry, as in all the physical sciences, is obtained initially from performing experiments in a laboratory. It is in the laboratory that facts are discovered and concepts, ideas and theorie ...
CH4 Student Revision Guides pdf | GCE AS/A
... This occurs when the isomers have the same molecular formula and same structural formula but have a different effect on plane polarised light. The isomers are non-superimposable mirror images. This property is known as chirality and the isomers are said to be chiral. Optical isomerism arises mainly ...
... This occurs when the isomers have the same molecular formula and same structural formula but have a different effect on plane polarised light. The isomers are non-superimposable mirror images. This property is known as chirality and the isomers are said to be chiral. Optical isomerism arises mainly ...
St. Xavier`s College – Autonomous Mumbai Syllabus for 3 Semester
... 1. To understand some more concepts of thermodynamics from a chemist’s viewpoint. 2. To predict the feasibility of a reaction. 3. To understand concepts involved in electrolytic cells and their applications. 4. To motivate students to solve numerical problems with different systems of units which il ...
... 1. To understand some more concepts of thermodynamics from a chemist’s viewpoint. 2. To predict the feasibility of a reaction. 3. To understand concepts involved in electrolytic cells and their applications. 4. To motivate students to solve numerical problems with different systems of units which il ...
Abstract - Engineering | UMass
... and without free residual chlorine. Other chlorination tests were run with solutions of aquatic NOM and treated drinking waters. DCAN concentrations were measured on all tests as were chlorine residuals. In some cases other DBPs were quantified too. Results indicate a decomposition scheme encompassi ...
... and without free residual chlorine. Other chlorination tests were run with solutions of aquatic NOM and treated drinking waters. DCAN concentrations were measured on all tests as were chlorine residuals. In some cases other DBPs were quantified too. Results indicate a decomposition scheme encompassi ...
Comparison between Free and Immobilized Ion
... van der Waals interaction is very weak (see Table 1), the attractive part of the PMF is largely due to the water-mediated hydrophobic interaction. Therefore, these two contact minima are both hydrophobic in nature. It is known that the thermodynamics of the hydrophobic hydration is size dependent.28 ...
... van der Waals interaction is very weak (see Table 1), the attractive part of the PMF is largely due to the water-mediated hydrophobic interaction. Therefore, these two contact minima are both hydrophobic in nature. It is known that the thermodynamics of the hydrophobic hydration is size dependent.28 ...
Net Ionic Equation Powerpoint Tutorial
... NH4OH H2O(l) + NH3(g) When one of these specific compounds forms, you just have to recognize it as one that undergoes this type of decomposition. By the way, only the first one would show visible bubbling and the CO2 gas forms. The other two don’t show bubbling: the gases are given off at the surf ...
... NH4OH H2O(l) + NH3(g) When one of these specific compounds forms, you just have to recognize it as one that undergoes this type of decomposition. By the way, only the first one would show visible bubbling and the CO2 gas forms. The other two don’t show bubbling: the gases are given off at the surf ...
17.2 The Avogadro Number
... Take another look at the chemical equation for making water: 2H 2 + O 2 → 2H 2 O Did you notice that something has been added? The large number in front of H2 tells how many molecules of H2 are required for the reaction to proceed. The large number in front of H2O tells how many molecules of water a ...
... Take another look at the chemical equation for making water: 2H 2 + O 2 → 2H 2 O Did you notice that something has been added? The large number in front of H2 tells how many molecules of H2 are required for the reaction to proceed. The large number in front of H2O tells how many molecules of water a ...
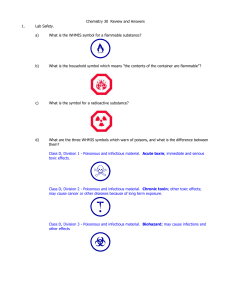
REVIEW and answers
... melting point (bottom right hand side of transition metals). As electrons become less delocalized the metals become harder, more brittle and conduct less well, but their melting and boiling points increase (top left hand side of transition metals). ...
... melting point (bottom right hand side of transition metals). As electrons become less delocalized the metals become harder, more brittle and conduct less well, but their melting and boiling points increase (top left hand side of transition metals). ...
Calculations from Balanced Equations
... Excess reactants You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reacti ...
... Excess reactants You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reacti ...
spontaneous change: entropy and free energy
... which depicts two identical glass bulbs joined by a stopcock. Initially, the bulb on the left contains an ideal gas at 1.00 atm pressure, and the bulb on the right is evacuated. When the valve is opened, the gas immediately expands into the evacuated bulb. After this expansion, the molecules are dis ...
... which depicts two identical glass bulbs joined by a stopcock. Initially, the bulb on the left contains an ideal gas at 1.00 atm pressure, and the bulb on the right is evacuated. When the valve is opened, the gas immediately expands into the evacuated bulb. After this expansion, the molecules are dis ...
Factors Controlling the Redox Activity of Oxygen in Perovskites
... coordination, is the prototypical iono-covalent structure. Due to the unique flexibility of this family in accepting a large variety of cations in A- or M-sites, the electronic conductivity of perovskites can be tuned from insulating to metallic, with numerous magnetic properties being reported [3]. ...
... coordination, is the prototypical iono-covalent structure. Due to the unique flexibility of this family in accepting a large variety of cations in A- or M-sites, the electronic conductivity of perovskites can be tuned from insulating to metallic, with numerous magnetic properties being reported [3]. ...
Solubility and Reactions
... 5. (a) The solubility of oxygen in blood is much greater than its solubility in pure water. Suggest a reason for this observation. (b) If the solubility of the oxygen in blood were the same as in pure water, how would your life be different? 318 Chapter 7 ...
... 5. (a) The solubility of oxygen in blood is much greater than its solubility in pure water. Suggest a reason for this observation. (b) If the solubility of the oxygen in blood were the same as in pure water, how would your life be different? 318 Chapter 7 ...
Introduction to Inorganic Chemistry
... order to achieve its ends. This means that a good chemist is one who not only has a mastery of chemical theory, but also a good knowledge of chemical facts. With such a knowledge, he can direct a trial and error approach to practical problems in the most promising directions. Inorganic Chemistry Org ...
... order to achieve its ends. This means that a good chemist is one who not only has a mastery of chemical theory, but also a good knowledge of chemical facts. With such a knowledge, he can direct a trial and error approach to practical problems in the most promising directions. Inorganic Chemistry Org ...
Solutions Manual
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
Modern inorganic chemistry
... We now know of the existence of over one hundred elements. A century ago, more than sixty of these were already known, and naturally attempts were made to relate the properties of all these elements in some way. One obvious method was to classify them as metals and non-metals; but this clearly did n ...
... We now know of the existence of over one hundred elements. A century ago, more than sixty of these were already known, and naturally attempts were made to relate the properties of all these elements in some way. One obvious method was to classify them as metals and non-metals; but this clearly did n ...
Chapter 23 Metals and Metallurgy
... • The electron-sea model does not explain observed trends in melting point, boiling point, heat of fusion, etc. – The model suggests these properties should increase with increasing number of valence ...
... • The electron-sea model does not explain observed trends in melting point, boiling point, heat of fusion, etc. – The model suggests these properties should increase with increasing number of valence ...
Introduction to Kinetics and Equilibrium
... In the equilibrium region, the concentrations of products and reactants ...
... In the equilibrium region, the concentrations of products and reactants ...
CB document - mvhs
... energy is released when new bonds form than was required to break existing bonds, then the difference will result in an overall release of energy. If, on the other hand, more energy is required to break existing bonds than is released when new bonds form, the difference will result overall in energy ...
... energy is released when new bonds form than was required to break existing bonds, then the difference will result in an overall release of energy. If, on the other hand, more energy is required to break existing bonds than is released when new bonds form, the difference will result overall in energy ...
laman web smk raja perempuan, ipoh
... 7. 5 Cell potentials from the combination of various electrode potentials; spontaneous and nonspontaneous electrode reactions ...
... 7. 5 Cell potentials from the combination of various electrode potentials; spontaneous and nonspontaneous electrode reactions ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.