Chem I Review Part 2
... 105.The C-N-O bond angle in nitromethane, CH3NO2, is expected to by approximately A. 60°. B. 90°. C. 109.5°. D. 120°. E. 180°. 106.Which one of the following molecules is nonpolar? A. NH3 B. OF2 C. CH3Cl D. H2O E. BeCl2 107.Complete this sentence: The PCl5 molecule has A. nonpolar bonds, and is a no ...
... 105.The C-N-O bond angle in nitromethane, CH3NO2, is expected to by approximately A. 60°. B. 90°. C. 109.5°. D. 120°. E. 180°. 106.Which one of the following molecules is nonpolar? A. NH3 B. OF2 C. CH3Cl D. H2O E. BeCl2 107.Complete this sentence: The PCl5 molecule has A. nonpolar bonds, and is a no ...
1. dia
... Chemistry of sulfur oxide formation • Sulfur trioxide at 482 oC transforms to sulfuric acid. Under the dew point sulfuric acid condensates on the structure materials (heat exchanger, stack wall ). The dew point of sulfuric acid depends on the SO3 and water content of the stack gas. Dew point of sul ...
... Chemistry of sulfur oxide formation • Sulfur trioxide at 482 oC transforms to sulfuric acid. Under the dew point sulfuric acid condensates on the structure materials (heat exchanger, stack wall ). The dew point of sulfuric acid depends on the SO3 and water content of the stack gas. Dew point of sul ...
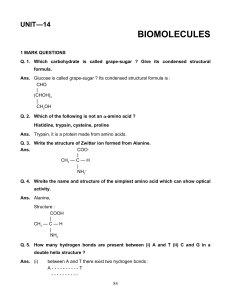
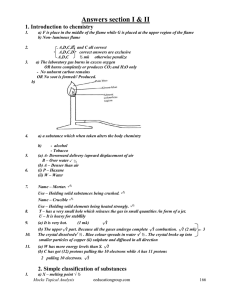
HOTS Worksheet
... Q. 1. When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed, what is the product obtained and what is its use in everyday life ? Ans. Aspirin used as analgesic. Q. 2. Distinguish between a narrow spectrum and broad spectrum antibiotic. Ans. A narrow spectrum antibiotic work ...
... Q. 1. When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed, what is the product obtained and what is its use in everyday life ? Ans. Aspirin used as analgesic. Q. 2. Distinguish between a narrow spectrum and broad spectrum antibiotic. Ans. A narrow spectrum antibiotic work ...
MEDICAL CHEMISTRY STUDY GUIDE
... Smoking is not allowed. Anyone who refuses to do so will be forced to leave the laboratory. Clothing and Footwear: Everyone must wear a lab coat during the lab and no shorts and sandals are allowed. Students who come to lab without proper clotting and shoes will be asked to go back for change. If t ...
... Smoking is not allowed. Anyone who refuses to do so will be forced to leave the laboratory. Clothing and Footwear: Everyone must wear a lab coat during the lab and no shorts and sandals are allowed. Students who come to lab without proper clotting and shoes will be asked to go back for change. If t ...
CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the 'kinetic model' of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the 'kinetic model' of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
Size-Selective Hydrogenation of Olefins by Dendrimer
... Because the maximum metal ion/dendrimer ratio is fixed for a particular metal ion, it follows that the maximum number of metal ions sorbed into the dendrimer is also nearly monodisperse and that the resulting nanoparticles are, likewise, of similar size. That is, the nanoparticle replica is a good r ...
... Because the maximum metal ion/dendrimer ratio is fixed for a particular metal ion, it follows that the maximum number of metal ions sorbed into the dendrimer is also nearly monodisperse and that the resulting nanoparticles are, likewise, of similar size. That is, the nanoparticle replica is a good r ...
Oxygen Isotope Effects as Structural and Mechanistic Probes in
... Interpretation of 18O EIEs. Klinman and co-workers were the first to determine 18O EIEs on the reversible binding of O2 to metalloproteins and formation of the oxygenated species in Table 1.24,37 These reactions involved reductive activation of O2 by FeII protoporphyrin IX in hemoglobin (Hb) and myo ...
... Interpretation of 18O EIEs. Klinman and co-workers were the first to determine 18O EIEs on the reversible binding of O2 to metalloproteins and formation of the oxygenated species in Table 1.24,37 These reactions involved reductive activation of O2 by FeII protoporphyrin IX in hemoglobin (Hb) and myo ...
PDF File
... group has no lone pair electrons and therefore cannot interact with a metal ion. The rSNH+3 reaction would therefore be expected to be severely compromised if the 2′-OH coordinates a metal ion important for catalysis (e.g. [10]). To determine the reactivity of rSNH+3 , it was necessary to establish ...
... group has no lone pair electrons and therefore cannot interact with a metal ion. The rSNH+3 reaction would therefore be expected to be severely compromised if the 2′-OH coordinates a metal ion important for catalysis (e.g. [10]). To determine the reactivity of rSNH+3 , it was necessary to establish ...
SQA CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the "kinetic model" of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the "kinetic model" of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
HEAd START TO A LEVEL CHEMISTRY WORKbOOK
... The masses of the various elements in a compound: eg 18 g of water, H 2 O, contains 2 g of hydrogen atoms and 16 g of oxygen since the relative atomic mass of hydrogen is 1 (x 2 because there two hydrogen atoms) and that of oxygen is 16. You should not learn large numbers of chemical formulae by hea ...
... The masses of the various elements in a compound: eg 18 g of water, H 2 O, contains 2 g of hydrogen atoms and 16 g of oxygen since the relative atomic mass of hydrogen is 1 (x 2 because there two hydrogen atoms) and that of oxygen is 16. You should not learn large numbers of chemical formulae by hea ...
AP Chemistry Curriculum Map - Belle Vernon Area School District
... excess reactants in chemical reactions. CHEM.B.2.1.2 – Use stoichiometric relationships to calculate the amounts of reactants and products involved in a chemical reaction. Standard: 3.2.C.A3 – Describe the three normal states of matter in terms of energy, particle motion, and phase ...
... excess reactants in chemical reactions. CHEM.B.2.1.2 – Use stoichiometric relationships to calculate the amounts of reactants and products involved in a chemical reaction. Standard: 3.2.C.A3 – Describe the three normal states of matter in terms of energy, particle motion, and phase ...
Reduction of CuO and Cu2O with H2: H Embedding
... (CH3OH + H2O w CO2 + 3H2),11 oxidative methanol reforming (CH3OH + 1/4O2 + 1/2H2O w CO2 + 5/2H2),12 NO reduction (NO + H2 w 1/2N2 + H2O),2b,13 etc. For years, there has been a big controversy about the relative importance of Cu+ and Cu0 centers in several of these catalytic reactions.2,9,12-15 The d ...
... (CH3OH + H2O w CO2 + 3H2),11 oxidative methanol reforming (CH3OH + 1/4O2 + 1/2H2O w CO2 + 5/2H2),12 NO reduction (NO + H2 w 1/2N2 + H2O),2b,13 etc. For years, there has been a big controversy about the relative importance of Cu+ and Cu0 centers in several of these catalytic reactions.2,9,12-15 The d ...
What is a mole? - Chemical Paradigms
... and dissolving it in a known volume of water. More about this later. In a chemical reaction substances need to combine in the correct mole ratio in order for the reaction to occur. Even if the number of moles changes the ratio of moles of reactants and products does not change. In the reaction below ...
... and dissolving it in a known volume of water. More about this later. In a chemical reaction substances need to combine in the correct mole ratio in order for the reaction to occur. Even if the number of moles changes the ratio of moles of reactants and products does not change. In the reaction below ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... new substance. Some common methods of measuring reaction rates involve the use of spectrometers, conductivity apparatus, and manometers (or a simple syringe). Note that concentration cannot be monitored directly. Emphasize that the observable (measurable) properties described in the following exampl ...
... new substance. Some common methods of measuring reaction rates involve the use of spectrometers, conductivity apparatus, and manometers (or a simple syringe). Note that concentration cannot be monitored directly. Emphasize that the observable (measurable) properties described in the following exampl ...
Chemistry 101L
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
Syllabus Advanced Level and Advanced Subsidiary Level
... Many of these Aims are reflected in the Assessment Objectives which follow; others are not readily assessed. The syllabus aims are to: ...
... Many of these Aims are reflected in the Assessment Objectives which follow; others are not readily assessed. The syllabus aims are to: ...
Isopropanol oxidation by pure metal oxide
... and NH3 . CO2 and NH3 do not measure all the surface sites. CO2 only adsorbs on basic OH groups on the surface and NH3 only adsorbs on Lewis and Bronsted acid sites. Furthermore, the above methods are unable to distinguish between surface acidic and redox sites. The adsorption and reaction of alcoho ...
... and NH3 . CO2 and NH3 do not measure all the surface sites. CO2 only adsorbs on basic OH groups on the surface and NH3 only adsorbs on Lewis and Bronsted acid sites. Furthermore, the above methods are unable to distinguish between surface acidic and redox sites. The adsorption and reaction of alcoho ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.