Production of materials
... in between; a chemical species formed between reactant(s) and product(s) ...
... in between; a chemical species formed between reactant(s) and product(s) ...
Chemistry Syllabus
... Methods of expressing concentration (use of normalities is not tested) Raoult’s law and colligative properties (nonvolatile solutes); osmosis Nonideal behavior (qualitative aspects) ...
... Methods of expressing concentration (use of normalities is not tested) Raoult’s law and colligative properties (nonvolatile solutes); osmosis Nonideal behavior (qualitative aspects) ...
Final Exam Study Guide Page 1 Quiz
... c. 1.5 x 1025 moles d. none of the above 2. How many grams are in 6.2 moles of NH4? a. .34 g b. 111.8 g c. 6.2 g d. 11.6 g 3. One mole of CaCO3 is equal to how many molecules of CaCO3? a. 765 molecules b. 249 molecules c. 7.6 x 1024 molecules d. 6.02 x 1023 molecules 4. How many grams of sodium are ...
... c. 1.5 x 1025 moles d. none of the above 2. How many grams are in 6.2 moles of NH4? a. .34 g b. 111.8 g c. 6.2 g d. 11.6 g 3. One mole of CaCO3 is equal to how many molecules of CaCO3? a. 765 molecules b. 249 molecules c. 7.6 x 1024 molecules d. 6.02 x 1023 molecules 4. How many grams of sodium are ...
im11
... and is used to clean the surface of metals, masonry, and cement. b) Vinegar is a dilute (5%) solution of acetic acid, a weak acid. c) Strong bases such as sodium hydroxide are used in drain cleaners. d) Ammonia, a weak base, dissolved in water is used as a household cleaner. 20. A mole is the name g ...
... and is used to clean the surface of metals, masonry, and cement. b) Vinegar is a dilute (5%) solution of acetic acid, a weak acid. c) Strong bases such as sodium hydroxide are used in drain cleaners. d) Ammonia, a weak base, dissolved in water is used as a household cleaner. 20. A mole is the name g ...
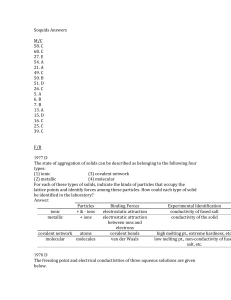
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... (d) solution 3, KMnO4 , ClO3– (e) solution 4, C2H5OH. Ethyl alcohol is covalently bonded and does not form ions in water. Therefore, the solution is not a better conductor of electricity than water, which is also covalently bonded. 2003 D Required For each of the following, use appropriate chemical ...
... (d) solution 3, KMnO4 , ClO3– (e) solution 4, C2H5OH. Ethyl alcohol is covalently bonded and does not form ions in water. Therefore, the solution is not a better conductor of electricity than water, which is also covalently bonded. 2003 D Required For each of the following, use appropriate chemical ...
Part A Completion
... ________ 8. The half-cell that has a greater tendency to acquire electrons will be the one in which oxidation occurs. ________ 9. In an electrochemical cell, the hydrogen half-cell is the reduction half-cell. ________ 10. A positive value for a standard reduction potential means hydrogen ions have a ...
... ________ 8. The half-cell that has a greater tendency to acquire electrons will be the one in which oxidation occurs. ________ 9. In an electrochemical cell, the hydrogen half-cell is the reduction half-cell. ________ 10. A positive value for a standard reduction potential means hydrogen ions have a ...
WEEK 6
... bond forms between the hydrogen ion and water molecule, yielding the hydronium ion. Both electrons in the bond were originally with the oxygen. This is different from most covalent bonds in which one electron comes from each of the atoms joined in the bond. To distinguish these two types of covalent ...
... bond forms between the hydrogen ion and water molecule, yielding the hydronium ion. Both electrons in the bond were originally with the oxygen. This is different from most covalent bonds in which one electron comes from each of the atoms joined in the bond. To distinguish these two types of covalent ...
AS 2, Organic, Physical and Inorganic Chemistry
... 12 Hess’s Law can be used to calculate enthalpy changes that cannot be measured by experiment. (a) State Hess’s Law. ...
... 12 Hess’s Law can be used to calculate enthalpy changes that cannot be measured by experiment. (a) State Hess’s Law. ...
Catalytic Synthesis of Organophosphorus Compounds from
... R′ = i-Pr, C6H11, PhCH2; R″ = Bu, C6H13, Ph. The reduced metals were recycled by the mixture of O 2-HNO2 (1), or NaBrO3 (2), C6H4O2 (3,4). It has been established that the PH 3 ligand is dissociated with formation of phosphide and an equivalent amount of acid inside coordination sphere of a high-val ...
... R′ = i-Pr, C6H11, PhCH2; R″ = Bu, C6H13, Ph. The reduced metals were recycled by the mixture of O 2-HNO2 (1), or NaBrO3 (2), C6H4O2 (3,4). It has been established that the PH 3 ligand is dissociated with formation of phosphide and an equivalent amount of acid inside coordination sphere of a high-val ...
Name: Northwest Vista College Chem 1311
... Reaction A: Reactants are at a higher energy level than products. 100 kJ of energy are required for activation and 100kJ are released. The reaction is exothermic Reaction B: Products are at a higher energy content than reactants. 250 kJ are required to activate the reaction. A total of 100 kJ are ab ...
... Reaction A: Reactants are at a higher energy level than products. 100 kJ of energy are required for activation and 100kJ are released. The reaction is exothermic Reaction B: Products are at a higher energy content than reactants. 250 kJ are required to activate the reaction. A total of 100 kJ are ab ...
pcc-sio2.alcohol.oxi..
... that is easily removed by the subsequent filtration. The celite/ silica gel residues are then deposited in the solid waste containers for disposal.1 While the conversion of cis,trans-4-tertbutylcyclohexanol to the corresponding ketone gives superior results in terms of an undergraduate protocol, oth ...
... that is easily removed by the subsequent filtration. The celite/ silica gel residues are then deposited in the solid waste containers for disposal.1 While the conversion of cis,trans-4-tertbutylcyclohexanol to the corresponding ketone gives superior results in terms of an undergraduate protocol, oth ...
Chemistry
... 4.3 Acids, Bases, and Neutralization Reactions. Acids, proton donors, monoprotoc acids, diprotic acids, bases, proton acceptors, hydroxides, strong and weak acids and bases, strong acids and bases as strong electrolytes, neutralization reactions, salts. 4.4 Oxidation-Reduction Reactions. Oxidation, ...
... 4.3 Acids, Bases, and Neutralization Reactions. Acids, proton donors, monoprotoc acids, diprotic acids, bases, proton acceptors, hydroxides, strong and weak acids and bases, strong acids and bases as strong electrolytes, neutralization reactions, salts. 4.4 Oxidation-Reduction Reactions. Oxidation, ...
Lectures on Chapter 4, Part 2 Powerpoint 97 Document
... SO32-(aq) SO42-(aq) + 2 e Add water to the reactant side to supply an oxygen and add two protons to the product side that will remain plus the two electrons. SO32-(aq) + H2O(l) SO42-(aq) + 2 H+(aq) + 2 e Reduction: MnO4-(aq) + 3 eMnO2 (s) Add water to the product side to take up the extra oxygen fro ...
... SO32-(aq) SO42-(aq) + 2 e Add water to the reactant side to supply an oxygen and add two protons to the product side that will remain plus the two electrons. SO32-(aq) + H2O(l) SO42-(aq) + 2 H+(aq) + 2 e Reduction: MnO4-(aq) + 3 eMnO2 (s) Add water to the product side to take up the extra oxygen fro ...
2012 C13 Exam answers
... This exam is being written by several thousand students. Please be sure that you follow the instructions below. We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News. ...
... This exam is being written by several thousand students. Please be sure that you follow the instructions below. We'll send your teacher a report on your performance. Top performers are eligible for a prize. The names of the top 200 students will be published in the September issue of Chem 13 News. ...
PIB and HH - Unit 4 - Chemical Names and Formulas
... 5. Which is larger, Au or Cu? 6. Which has greater ionization energy, Cu or Ag? 7. Which has greater shielding, Xe or Ar? 8. Which is larger, Ca or Cs? 9. Which has greater shielding, Se or Ra? 10. Which has greater nuclear charge, Zn or Se? 11. Which is larger, Mg or P? 12. Which has greater ioniza ...
... 5. Which is larger, Au or Cu? 6. Which has greater ionization energy, Cu or Ag? 7. Which has greater shielding, Xe or Ar? 8. Which is larger, Ca or Cs? 9. Which has greater shielding, Se or Ra? 10. Which has greater nuclear charge, Zn or Se? 11. Which is larger, Mg or P? 12. Which has greater ioniza ...
Chemistry-5th-Edition-Brady-Solution-Manual
... The heavy line separates the metals from the nonmetals, and the metalloids border the line. ...
... The heavy line separates the metals from the nonmetals, and the metalloids border the line. ...
Active metamaterials with negative static dielectric susceptibility
... While this procedure results in an experimentally determined value of χz that is clearly negative, the result must be considered preliminary in the respect that it concerns only a single unit cell for which the necessary circuitry and power supply are implemented externally. Further experiments are ...
... While this procedure results in an experimentally determined value of χz that is clearly negative, the result must be considered preliminary in the respect that it concerns only a single unit cell for which the necessary circuitry and power supply are implemented externally. Further experiments are ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.