Chemistry Final Exam Review
... 3. If you have a 2.75dm3 balloon under 455kPa of pressure at 100oC, what will the volume be at STP? ...
... 3. If you have a 2.75dm3 balloon under 455kPa of pressure at 100oC, what will the volume be at STP? ...
Summer_Assignment_AP_Chemistry_TW 2015
... Watch your stoichiometry. A lot of problems will be set up perfectly within the problem itself, so keep your eyes peeled. Memorize strong acids and bases. They ionize 100%, and are therefore very important. It's okay if you don't get something right the first time. Try again and again, until you can ...
... Watch your stoichiometry. A lot of problems will be set up perfectly within the problem itself, so keep your eyes peeled. Memorize strong acids and bases. They ionize 100%, and are therefore very important. It's okay if you don't get something right the first time. Try again and again, until you can ...
Document
... time to each side. lNow they are forced to pair up. lWe have now written the electron dot ...
... time to each side. lNow they are forced to pair up. lWe have now written the electron dot ...
Chemistry Spell check on
... 1 Check that the answer sheet provided is for Chemistry Higher (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Ce ...
... 1 Check that the answer sheet provided is for Chemistry Higher (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Ce ...
51 Draw a Lewis electron-dot diagram for a
... 59 Identify the class of organic compounds to which ethanol belongs. [1] 60 A liquid boils when the vapor pressure of the liquid equals the atmospheric pressure on the surface of the liquid. Based on Table H, what is the boiling point of ethanol at standard pressure? [1] 61 Compare the intermolecula ...
... 59 Identify the class of organic compounds to which ethanol belongs. [1] 60 A liquid boils when the vapor pressure of the liquid equals the atmospheric pressure on the surface of the liquid. Based on Table H, what is the boiling point of ethanol at standard pressure? [1] 61 Compare the intermolecula ...
Document
... 1 Check that the answer sheet provided is for Chemistry Higher (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Ce ...
... 1 Check that the answer sheet provided is for Chemistry Higher (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Ce ...
About Magnetism - Georgetown College
... permanent magnets can lose their magnetic fields, which is why refrigerator magnets stop working after a few years.] In honor of iron, these materials are called “ferromagnetic” (fer is Latin for iron). Most materials are not pure substances, but rather compounds made of more than one element. While ...
... permanent magnets can lose their magnetic fields, which is why refrigerator magnets stop working after a few years.] In honor of iron, these materials are called “ferromagnetic” (fer is Latin for iron). Most materials are not pure substances, but rather compounds made of more than one element. While ...
Supplement AP Chemistry –
... (d) How many millimoles of solid NaOH must be added to 50.0 milliliters of 0.20- molar HOCl to obtain a buffer solution that has a pH of 7.49? Assume that the addition of solid NaOH results in a negligible change in volume. (e) Household bleach is made by dissolving chlorine gas in water, as represe ...
... (d) How many millimoles of solid NaOH must be added to 50.0 milliliters of 0.20- molar HOCl to obtain a buffer solution that has a pH of 7.49? Assume that the addition of solid NaOH results in a negligible change in volume. (e) Household bleach is made by dissolving chlorine gas in water, as represe ...
Chemistry - NIC Karnataka
... + SO3(aq) Cr 3aq + SO24(aq) (acidic medium) Cr2O7(aq) MnO4–(aq) + Br–(aq) MnO2(s)+BrO3–(aq)(acidic medium) b) Half reaction method : Fe2+(aq)+Cr2O72–(aq) Fe3+(aq)+Cr3+aq) (acidic medium) MnO4–(aq) + I–(aq) MnO2(s) + I2(s) (basic medium) Applications: redox titrations, redox indicators - with example ...
... + SO3(aq) Cr 3aq + SO24(aq) (acidic medium) Cr2O7(aq) MnO4–(aq) + Br–(aq) MnO2(s)+BrO3–(aq)(acidic medium) b) Half reaction method : Fe2+(aq)+Cr2O72–(aq) Fe3+(aq)+Cr3+aq) (acidic medium) MnO4–(aq) + I–(aq) MnO2(s) + I2(s) (basic medium) Applications: redox titrations, redox indicators - with example ...
File - chemistryattweed
... and was interested in the effect of heat on the chemistry of gases. In the early 1900s, Haber reacted nitrogen with hydrogen, using an iron catalyst, to form ammonia. Ammonia can be readily converted to a range of valuable products. In 1908 he had improved the reaction and in 1911 he was rewarded wi ...
... and was interested in the effect of heat on the chemistry of gases. In the early 1900s, Haber reacted nitrogen with hydrogen, using an iron catalyst, to form ammonia. Ammonia can be readily converted to a range of valuable products. In 1908 he had improved the reaction and in 1911 he was rewarded wi ...
File
... VIII. For the reaction H2(g) + Cl2(g) 2 HCl(g), ΔH̊ = –184.6 kJ. Use the information above and the information on the accompanying chart to find the value of ΔS̊ for the reaction given. (at 298 K) ( 3 pts) IX. A standard chemical cell is constructed using 1.00 molar solutions of Ni(NO3)2 and zinc ...
... VIII. For the reaction H2(g) + Cl2(g) 2 HCl(g), ΔH̊ = –184.6 kJ. Use the information above and the information on the accompanying chart to find the value of ΔS̊ for the reaction given. (at 298 K) ( 3 pts) IX. A standard chemical cell is constructed using 1.00 molar solutions of Ni(NO3)2 and zinc ...
Inorganic Chemistry 412 / 512
... The inert pair effect. Pb forms weaker bonds than Sn, and the highest oxidation state is therefore less stable. ...
... The inert pair effect. Pb forms weaker bonds than Sn, and the highest oxidation state is therefore less stable. ...
AP Chemistry - cloudfront.net
... (c) What curve corresponds to the conditions at which the solid and gas are in equilibrium? (d) Describe what happens when you start at point H and decrease the pressure at constant temperature. (f) is liquid X more or less dense than solid X? 11.108 The density of solid gallium at its melting point ...
... (c) What curve corresponds to the conditions at which the solid and gas are in equilibrium? (d) Describe what happens when you start at point H and decrease the pressure at constant temperature. (f) is liquid X more or less dense than solid X? 11.108 The density of solid gallium at its melting point ...
Presentation by class of 2013
... equilibrium that takes place in a closed system when the rate of the forward and backward reaction is equal. A closed system means no atoms of the products or reactants can escape to the outside environment (i.e. a ...
... equilibrium that takes place in a closed system when the rate of the forward and backward reaction is equal. A closed system means no atoms of the products or reactants can escape to the outside environment (i.e. a ...
Year End Review
... 13. During a chemical reaction which of the following statements is true? a. When one of the products of reaction is a gas the mass of the reactants will be greater than the mass of the products. b. When of the products is a precipitate the mass of the products will be greater than the mass of the r ...
... 13. During a chemical reaction which of the following statements is true? a. When one of the products of reaction is a gas the mass of the reactants will be greater than the mass of the products. b. When of the products is a precipitate the mass of the products will be greater than the mass of the r ...
Chemistry Spell check on
... 12. A student was asked to carry out an experiment to determine the concentration of a copper(II) sulphate solution. Part of the work card used is shown. ...
... 12. A student was asked to carry out an experiment to determine the concentration of a copper(II) sulphate solution. Part of the work card used is shown. ...
odd - WWW2
... The first point of contrast is the difference in boiling points: the nonpolar hydrocarbons have very low boiling points, while the hydrogen-bonding hydrides of nitrogen have much higher boiling points. Following from this, they have different acid-base properties, both hydrides of carbon being neutr ...
... The first point of contrast is the difference in boiling points: the nonpolar hydrocarbons have very low boiling points, while the hydrogen-bonding hydrides of nitrogen have much higher boiling points. Following from this, they have different acid-base properties, both hydrides of carbon being neutr ...
Document
... proton-transfer reactions to justify the identification. [See SP 6.1; Essential knowledge 3.B.2] Learning objective 3.8 The student is able to identify redox reactions and justify the identification in terms of electron transfer. [See SP 6.1; Essential knowledge 3.B.3] Learning objective 3.9 The stu ...
... proton-transfer reactions to justify the identification. [See SP 6.1; Essential knowledge 3.B.2] Learning objective 3.8 The student is able to identify redox reactions and justify the identification in terms of electron transfer. [See SP 6.1; Essential knowledge 3.B.3] Learning objective 3.9 The stu ...
PVS103 - unit 6 notes
... • Boron is unique in the group in that it is clearly a non-metal, we will concentrate on its properties, as it is very interesting. • The molecules boron forms are unique in that they do not conform fully to Lewis theory, for instance BH3 is a stable molecule, but there is no octet of electrons on b ...
... • Boron is unique in the group in that it is clearly a non-metal, we will concentrate on its properties, as it is very interesting. • The molecules boron forms are unique in that they do not conform fully to Lewis theory, for instance BH3 is a stable molecule, but there is no octet of electrons on b ...
unit_k_reading_notes
... (atm) = 760 mm Hg. Standard temperature and pressure together are designated as STP. You don’t have to convert between these values at this time, but you need to be able to recognize that they are standard temperature and pressure. Avogadro’s Hypothesis (or Law) states that equal volumes of any two ...
... (atm) = 760 mm Hg. Standard temperature and pressure together are designated as STP. You don’t have to convert between these values at this time, but you need to be able to recognize that they are standard temperature and pressure. Avogadro’s Hypothesis (or Law) states that equal volumes of any two ...
Production of materials
... in between; a chemical species formed between reactant(s) and product(s) ...
... in between; a chemical species formed between reactant(s) and product(s) ...
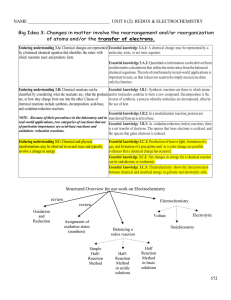
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.