Notes - Text
... If we start with a mixture of nitrogen and hydrogen (in any proportions), the reaction will reach equilibrium with constant concentrations of nitrogen, hydrogen, and ammonia. However, if we start with just ammonia and no nitrogen or hydrogen, the reaction will proceed and N2 and H2 will be produced ...
... If we start with a mixture of nitrogen and hydrogen (in any proportions), the reaction will reach equilibrium with constant concentrations of nitrogen, hydrogen, and ammonia. However, if we start with just ammonia and no nitrogen or hydrogen, the reaction will proceed and N2 and H2 will be produced ...
Structurally diagnostic ion/molecule reactions
... In CI, however, ion formation and its further reaction are performed in the same region (ion source) without previous ion isolation; hence the desired reaction may occur concurrently with other reactions involving cogenerated ions. With the development of MS instrumentation, particularly after the i ...
... In CI, however, ion formation and its further reaction are performed in the same region (ion source) without previous ion isolation; hence the desired reaction may occur concurrently with other reactions involving cogenerated ions. With the development of MS instrumentation, particularly after the i ...
Microsoft Word - Ethesis@nitr
... express my deep sense of gratitude and indebtedness to Prof. Niranjan Panda, Department of Chemistry, National Institute of Technology, Rourkela, for introducing the present project topic and for her inspiring guidance, constructive criticism and valuable suggestion throughout the project work. I mo ...
... express my deep sense of gratitude and indebtedness to Prof. Niranjan Panda, Department of Chemistry, National Institute of Technology, Rourkela, for introducing the present project topic and for her inspiring guidance, constructive criticism and valuable suggestion throughout the project work. I mo ...
File
... Examples of chemical systems in equilibrium: 1. Solubility Equilibrium • in solubility equilibrium the ________ solute particles continuously dissolve into solution, while an equal number of dissolved solute particles in solution crystallize or _________ out of solution 2. Phase Equilibrium - in ph ...
... Examples of chemical systems in equilibrium: 1. Solubility Equilibrium • in solubility equilibrium the ________ solute particles continuously dissolve into solution, while an equal number of dissolved solute particles in solution crystallize or _________ out of solution 2. Phase Equilibrium - in ph ...
AQA GCSE Chemistry My Revision Notes
... (b) Give the names and formulae of the two ions that can make water hard. (4 marks) (c) There are some advantages of drinking hard water. Give one of them. (1 mark) (d) What happens if you use temporarily hard water in a kettle? (2 marks) (e) Explain how an ion-exchange column softens hard water. (2 ...
... (b) Give the names and formulae of the two ions that can make water hard. (4 marks) (c) There are some advantages of drinking hard water. Give one of them. (1 mark) (d) What happens if you use temporarily hard water in a kettle? (2 marks) (e) Explain how an ion-exchange column softens hard water. (2 ...
Some uses of mischmetall in organic synthesis
... Results and discussion Mischmetall as a coreductant in samarium(II) catalysed reactions Since its introduction in synthetic organic chemistry in 1977,1 samarium diiodide has become one of the most popular reagents. However, its cost could be considered as a major drawback, potentially limiting the d ...
... Results and discussion Mischmetall as a coreductant in samarium(II) catalysed reactions Since its introduction in synthetic organic chemistry in 1977,1 samarium diiodide has become one of the most popular reagents. However, its cost could be considered as a major drawback, potentially limiting the d ...
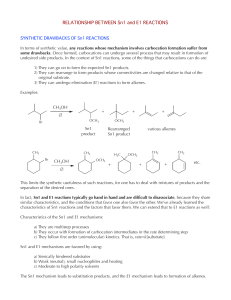
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... H Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat ...
... H Why is the trisubstituted alkene more stable than the other two? The relative stabilities of alkenes is measured by their heat of hydrogenation (much like the relative stabilities of cycloalkanes is measured by their heats of combustion, see ch. 3). The relationship is inverse: the higher the heat ...
ALDEHYDES & KETONES - Rogue Community College
... Aliphatic chains NOT as part of ... Aliphatic rings or Aromatic rings ...
... Aliphatic chains NOT as part of ... Aliphatic rings or Aromatic rings ...
A mechanistic approach to solvolysis of n-caproyl chloride (n
... Raveendran & Jayasree, Orient. J. Chem., Vol. 25(4), 1017-1022 (2009) All these obser vations lead to the conclusion that hydrolysis in benzoyl chloride and to a larger extent in acetyl chloride proceeds largely by SN2 mechanism with simultaneous involvement of addition-ionization mechanism propose ...
... Raveendran & Jayasree, Orient. J. Chem., Vol. 25(4), 1017-1022 (2009) All these obser vations lead to the conclusion that hydrolysis in benzoyl chloride and to a larger extent in acetyl chloride proceeds largely by SN2 mechanism with simultaneous involvement of addition-ionization mechanism propose ...
Document
... • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one stereoisomer is formed preferentially. Why? ...
... • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one stereoisomer is formed preferentially. Why? ...
chemistry writing team
... Law of conservation of mass : ‘Mass can neither be created nor destroyed.’ In all physical and chemical changes, the total mass of reactants is equal to that of products. Law of constant composition : A chemical compound is always found to be made of same elements combined together in the same fixed ...
... Law of conservation of mass : ‘Mass can neither be created nor destroyed.’ In all physical and chemical changes, the total mass of reactants is equal to that of products. Law of constant composition : A chemical compound is always found to be made of same elements combined together in the same fixed ...
Organic Chemistry
... Addition of Cl2 and Br2 • for a cyclohexene, anti coplanar addition corresponds to trans diaxial addition • the initial trans diaxial conformation is in equilibrium with the more stable trans diequatorial conformation • because the bromonium ion can form on either face of the alkene with equal prob ...
... Addition of Cl2 and Br2 • for a cyclohexene, anti coplanar addition corresponds to trans diaxial addition • the initial trans diaxial conformation is in equilibrium with the more stable trans diequatorial conformation • because the bromonium ion can form on either face of the alkene with equal prob ...
Pincer Complexes. Applications in Catalysis
... In 1976 Moulton and Shaw reported [1] for the first time the synthesis of the complex IrHCl{C6H3-2,6-(CH2PBut2)2} (11). They observed that this compound exhibited a high thermal stability, subliming without visible decomposition at temperatures as high as 180 oC. These results, led independently to ...
... In 1976 Moulton and Shaw reported [1] for the first time the synthesis of the complex IrHCl{C6H3-2,6-(CH2PBut2)2} (11). They observed that this compound exhibited a high thermal stability, subliming without visible decomposition at temperatures as high as 180 oC. These results, led independently to ...