Topic 1: Chemical Reactions
... commonplace everyday occurrences: the burning of gas in a cooker, the rusting of cars, the hardening of glues, the digestion of food and so on. Reference should also be made to the work of chemists in developing materials which affect our way of life, although great care needs to be exercised in wid ...
... commonplace everyday occurrences: the burning of gas in a cooker, the rusting of cars, the hardening of glues, the digestion of food and so on. Reference should also be made to the work of chemists in developing materials which affect our way of life, although great care needs to be exercised in wid ...
AP Chemistry
... trend Lattice energy % ionic character Lewis structures Valence electrons Octet rule Resonance Formal charge VSEPR model Bond angles Hybridization , bonds ...
... trend Lattice energy % ionic character Lewis structures Valence electrons Octet rule Resonance Formal charge VSEPR model Bond angles Hybridization , bonds ...
Chapter 11
... reactants to the left of the arrow separated by plus signs and the products to the right of the arrow separated by plus signs. ► Word equation- elements or compounds are represented by words Example – Iron + oxygen Iron(III)oxide ...
... reactants to the left of the arrow separated by plus signs and the products to the right of the arrow separated by plus signs. ► Word equation- elements or compounds are represented by words Example – Iron + oxygen Iron(III)oxide ...
Unit 14.1 REDOX Reactions Objectives REDOX Reactions
... • REDOX reactions involve the transfer of electrons from one species to another. • A REDOX reaction involves both an oxidation of one species and a reduction of another. • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
... • REDOX reactions involve the transfer of electrons from one species to another. • A REDOX reaction involves both an oxidation of one species and a reduction of another. • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
Name
... (b)Given the photoelectron spectra below for phosphorus, P, and sulfur, S, which of the following best explains why the 2p peak for S is further to the left than the 2p peak for P, but the 3p peak for S is further to the right than the 3p peak for P? Circle your answer. I. S has a greater effective ...
... (b)Given the photoelectron spectra below for phosphorus, P, and sulfur, S, which of the following best explains why the 2p peak for S is further to the left than the 2p peak for P, but the 3p peak for S is further to the right than the 3p peak for P? Circle your answer. I. S has a greater effective ...
Chemical Equations and Reactions
... with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
... with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
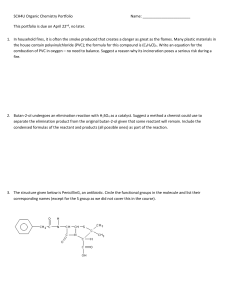
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
Chemistry Comes Alive: Part A
... • Unequal sharing by atoms with different electron-attracting abilities produces polar molecules • H2O • Atoms with six or seven valence shell electrons are electronegative, e.g., oxygen • Atoms with one or two valence shell electrons are electropositive, e.g., sodium ...
... • Unequal sharing by atoms with different electron-attracting abilities produces polar molecules • H2O • Atoms with six or seven valence shell electrons are electronegative, e.g., oxygen • Atoms with one or two valence shell electrons are electropositive, e.g., sodium ...
Regents_Chem_Core_for_review
... IV.2 Two major categories of compounds are ionic and molecular (covalent) compounds. (5.2g) IV.3 Chemical bonds are formed when valence electrons are (5.2a): • transferred from one atom to another (ionic) • shared between atoms (covalent) • mobile within a metal (metallic) IV.4 In a multiple covalen ...
... IV.2 Two major categories of compounds are ionic and molecular (covalent) compounds. (5.2g) IV.3 Chemical bonds are formed when valence electrons are (5.2a): • transferred from one atom to another (ionic) • shared between atoms (covalent) • mobile within a metal (metallic) IV.4 In a multiple covalen ...
Thermodynamics
... DV is the volume change involved in the reaction (Vwater - Vice) similarly DS and DG are the entropy and free energy changes dDG is then the change in DG as T and P are varied ...
... DV is the volume change involved in the reaction (Vwater - Vice) similarly DS and DG are the entropy and free energy changes dDG is then the change in DG as T and P are varied ...
Chemistry C2 Part One
... • So, an increase in the pressure shifts the equilibrium to the right and increases the yield of ammonia • A high pressure of 100 - 200 atmospheres is chosen for the Haber process • Using higher pressures adds to building costs (walls for reaction chambers will be thicker) and running costs (faster ...
... • So, an increase in the pressure shifts the equilibrium to the right and increases the yield of ammonia • A high pressure of 100 - 200 atmospheres is chosen for the Haber process • Using higher pressures adds to building costs (walls for reaction chambers will be thicker) and running costs (faster ...