Molar Heat of Reaction
... Miss Mullin’s Go-To Answer for Inorganic Chemistry in CEGEP (not a real title) ...
... Miss Mullin’s Go-To Answer for Inorganic Chemistry in CEGEP (not a real title) ...
SF Chemical Kinetics Michaelmas 2011 L5
... Potential energy surface: 3-D plot of the energy of all possible arrangements of the atoms in an activated complex. Defines the easiest route (the col between regions of high energy ) and hence the exact position of the transition state. For the simplest case of the reaction of two structureless par ...
... Potential energy surface: 3-D plot of the energy of all possible arrangements of the atoms in an activated complex. Defines the easiest route (the col between regions of high energy ) and hence the exact position of the transition state. For the simplest case of the reaction of two structureless par ...
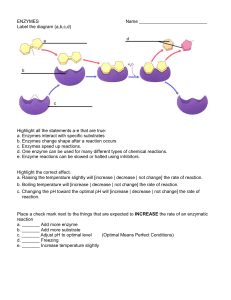
ENZYMES
... Place a check mark next to the things that are expected to INCREASE the rate of an enzymatic reaction a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... Place a check mark next to the things that are expected to INCREASE the rate of an enzymatic reaction a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
Chapter 2
... Solute particles are larger than in a solution and scatter light; do not settle out. ...
... Solute particles are larger than in a solution and scatter light; do not settle out. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 18. Explain the mechanism of markownikoff and antimarkownikoff addition of propene. 19. Write short notes on a)hydroboration reaction b) addition polymerization reaction 20. Explain the mode of hybridization of carbon in methane, ethylene and acetylene. 21. Explain cis and trans addition with an exa ...
... 18. Explain the mechanism of markownikoff and antimarkownikoff addition of propene. 19. Write short notes on a)hydroboration reaction b) addition polymerization reaction 20. Explain the mode of hybridization of carbon in methane, ethylene and acetylene. 21. Explain cis and trans addition with an exa ...
EXAM REVIEW !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! The examination is scheduled
... Writing out a differential given information on its variables. Calculations to find G at a different temperature Calculations to find G at a different pressure Use of the definition of the Chemical Potentials Expression for the molar Gibbs free energy of a gas Expression for the Fundamental Eqn of ...
... Writing out a differential given information on its variables. Calculations to find G at a different temperature Calculations to find G at a different pressure Use of the definition of the Chemical Potentials Expression for the molar Gibbs free energy of a gas Expression for the Fundamental Eqn of ...
Just a Few Things 2012
... In BOTH types of cell the same types of reaction occur at the same electrode: ANode — OXidation ...
... In BOTH types of cell the same types of reaction occur at the same electrode: ANode — OXidation ...
Thermodynamics and kinetics
... • At equilibrium, no further change as long as external conditions are constant • Change in external conditions can change equilibrium A stressed system at equilibrium will shift to reduce stress concentration, pressure, temperature • N2 + 3 H2 <--> 2 NH3 + 22 kcal What is the shift due to Inc ...
... • At equilibrium, no further change as long as external conditions are constant • Change in external conditions can change equilibrium A stressed system at equilibrium will shift to reduce stress concentration, pressure, temperature • N2 + 3 H2 <--> 2 NH3 + 22 kcal What is the shift due to Inc ...
1) Which of the following correctly lists the atoms in order of
... 1) Which of the following correctly lists the atoms in order of increasing size (smallest to largest)? a) F< K < Ge < Br < Rb b) F < Br < Ge < K < Rb c) F < Ge < Br < K < Rb d) F < K < Br < Ge < Rb e) F < Br < Ge < Rb < K 2). The first six ionization energies for an element are: 786 kJ, 1580 kJ, 323 ...
... 1) Which of the following correctly lists the atoms in order of increasing size (smallest to largest)? a) F< K < Ge < Br < Rb b) F < Br < Ge < K < Rb c) F < Ge < Br < K < Rb d) F < K < Br < Ge < Rb e) F < Br < Ge < Rb < K 2). The first six ionization energies for an element are: 786 kJ, 1580 kJ, 323 ...
Stoichiometry
... Incomplete – occurs when there is a limited amount of oxygen CO(g) + H2O(l) ...
... Incomplete – occurs when there is a limited amount of oxygen CO(g) + H2O(l) ...
Course Syllabus - Honors Chemistry
... b. Chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. c. Salt crystals, such as NaCl, are repeating patterns of positive and negative ions held together by electrostatic attraction. d. Atoms and molecules in liquids move ...
... b. Chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. c. Salt crystals, such as NaCl, are repeating patterns of positive and negative ions held together by electrostatic attraction. d. Atoms and molecules in liquids move ...
Chemical Equations & Reactions
... and/or formulas for the reactants and products Can be either a word equation or a formula equation The law of conservation of mass must be satisfied. This provides the basis for balancing chemical equations. 1st formulated by Antoine Lavoisier ...
... and/or formulas for the reactants and products Can be either a word equation or a formula equation The law of conservation of mass must be satisfied. This provides the basis for balancing chemical equations. 1st formulated by Antoine Lavoisier ...
Slide 1
... Some chemical and physical changes take place by themselves, given enough time. A spontaneous chemical reaction is one that, given sufficient time, will achieve chemical equilibrium, with an equilibrium constant greater than 1, by reacting from left to right. ...
... Some chemical and physical changes take place by themselves, given enough time. A spontaneous chemical reaction is one that, given sufficient time, will achieve chemical equilibrium, with an equilibrium constant greater than 1, by reacting from left to right. ...