FREE RADICAL REACTIONS IN ORGANIC SYNTHESIS
... MECHANISM • Before we look at free radical chemistry a quick revision of mechanisms (again) • You should all be happy with curly arrow represents the movement of 2 e– ...
... MECHANISM • Before we look at free radical chemistry a quick revision of mechanisms (again) • You should all be happy with curly arrow represents the movement of 2 e– ...
Carbene Singlets, Triplets, and the Physics that
... while knowing a set of rules for writing them allows them to be consistent with experiments in cases where there are no extremely similar energy levels in the interaction diagram, they are not suitable for discerning numerical results. However, as will be shown in more detail later, these molecular ...
... while knowing a set of rules for writing them allows them to be consistent with experiments in cases where there are no extremely similar energy levels in the interaction diagram, they are not suitable for discerning numerical results. However, as will be shown in more detail later, these molecular ...
Elimination Reactions
... • Now the double bond of the alkene is fully formed and the alkene has a trigonal plannar geometry at each carbon atom. The other products are a molecule of ethanol and a bromide ion. ...
... • Now the double bond of the alkene is fully formed and the alkene has a trigonal plannar geometry at each carbon atom. The other products are a molecule of ethanol and a bromide ion. ...
Redox Reactions - Hillsborough County Public Schools
... A compound has an overall charge of zero, which means all the negative charges have to equal the positive charges. Examples: When calculating the oxidation number of N in NO2 , use the rules above to help you. You see that oxygen normally has an oxidation number of -2 and there are two oxygen atoms. ...
... A compound has an overall charge of zero, which means all the negative charges have to equal the positive charges. Examples: When calculating the oxidation number of N in NO2 , use the rules above to help you. You see that oxygen normally has an oxidation number of -2 and there are two oxygen atoms. ...
SAMPLE PAPER -9 Time Allowed: 3 Hrs
... 18. Account for the following 1. HCHO gives Cannizaro’s reaction . Where as CH3CHO doesn’t 2. Write chemical test to distinguish Phenol and benzoic acid. 19. What happens when a. Beam of light is passed through Fe(OH)3 solution b. KCl is added to Fe(OH)3s c. River and Sea meet 20. Acompound A is ext ...
... 18. Account for the following 1. HCHO gives Cannizaro’s reaction . Where as CH3CHO doesn’t 2. Write chemical test to distinguish Phenol and benzoic acid. 19. What happens when a. Beam of light is passed through Fe(OH)3 solution b. KCl is added to Fe(OH)3s c. River and Sea meet 20. Acompound A is ext ...
Working With Chemical Reactions
... Unfortunately some characteristic reactions do need to be recognized and memorized. Carbon disulfide has a characteristic reaction with oxygen to produce Carbon Dioxide and Sulfur ...
... Unfortunately some characteristic reactions do need to be recognized and memorized. Carbon disulfide has a characteristic reaction with oxygen to produce Carbon Dioxide and Sulfur ...
Document
... The –OH group of an alcohol must be converted into a better leaving group for alcohols to participate in substitution or elimination reactions. ...
... The –OH group of an alcohol must be converted into a better leaving group for alcohols to participate in substitution or elimination reactions. ...
Document
... 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O2-, is –½. ...
... 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O2-, is –½. ...
ap-thermochemistry - Waukee Community School District Blogs
... same, but its sign changes. When the balanced equation for a reaction is multiplied by an integer, the value of ΔH for that reaction must be multiplied by the ...
... same, but its sign changes. When the balanced equation for a reaction is multiplied by an integer, the value of ΔH for that reaction must be multiplied by the ...
Whitten, Davis, and Peck, General Chemistry, 6th Edition
... Recommended CER Experiments to accompany Hornback’s Organic Chemistry, Second Edition The table below matches sections from the book with recommended CER labs. Click on the experiment title to view a PDF of each lab. Go to www.CERLabs.com to search the complete CER database and to learn more about c ...
... Recommended CER Experiments to accompany Hornback’s Organic Chemistry, Second Edition The table below matches sections from the book with recommended CER labs. Click on the experiment title to view a PDF of each lab. Go to www.CERLabs.com to search the complete CER database and to learn more about c ...
Basic chemistry - Ross University
... to measure changes in enthalpy. For biochemical reactions we can thus use ∆H as an approximation for ∆E, which is the much more important parameter. Entropy (S) is a measure of chaos. If you look at fig. 1.1, you see two bodies of different temperature. If you bring them close together, heat could t ...
... to measure changes in enthalpy. For biochemical reactions we can thus use ∆H as an approximation for ∆E, which is the much more important parameter. Entropy (S) is a measure of chaos. If you look at fig. 1.1, you see two bodies of different temperature. If you bring them close together, heat could t ...
ch437 class 18
... on the molecular orbital energies, which in turn depend upon the structure of the chromophore. It is usually very difficult to give accurate prediction purely from theory how absorption changes as the chromophore structure changes. Instead, empirical rules (Class 20) are much more conveniently used ...
... on the molecular orbital energies, which in turn depend upon the structure of the chromophore. It is usually very difficult to give accurate prediction purely from theory how absorption changes as the chromophore structure changes. Instead, empirical rules (Class 20) are much more conveniently used ...
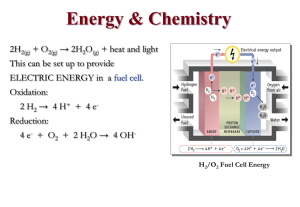
lec03 - McMaster Chemistry
... Energy is released as HEAT, LIGHT, SOUND, WORK • if more energy is used in breaking bonds than is released in forming bonds then . . . reaction is ENDOTHERMIC ...
... Energy is released as HEAT, LIGHT, SOUND, WORK • if more energy is used in breaking bonds than is released in forming bonds then . . . reaction is ENDOTHERMIC ...