1 - kurtniedenzu
... a. More protons b. Fewer protons c. More neutrons d. Fewer neutrons 3. How many electrons are present in the electron-dot diagram of an atom with atomic number 9? a. 2 b. 7 c. 9 d. 11 4. If atom X is represented by 126X and atom Y is represented by 146Y, then X and Y are: a. isotopes of the same ele ...
... a. More protons b. Fewer protons c. More neutrons d. Fewer neutrons 3. How many electrons are present in the electron-dot diagram of an atom with atomic number 9? a. 2 b. 7 c. 9 d. 11 4. If atom X is represented by 126X and atom Y is represented by 146Y, then X and Y are: a. isotopes of the same ele ...
Thermochemistry
... Another form of the first law for DEsystem DE = q + w DE is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PDV when a gas expands against a constant external pressure ...
... Another form of the first law for DEsystem DE = q + w DE is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PDV when a gas expands against a constant external pressure ...
Slide 1
... oxidation is defined as the ____ loss of ________ _____ of a ________ electrons from the atoms substance -during its reaction with Chlorine _______, Sodium ______ _____ loses ________: electrons oxidation number is equal to the number of electrons lost or gained by an atom Reduction causes the numer ...
... oxidation is defined as the ____ loss of ________ _____ of a ________ electrons from the atoms substance -during its reaction with Chlorine _______, Sodium ______ _____ loses ________: electrons oxidation number is equal to the number of electrons lost or gained by an atom Reduction causes the numer ...
Subject Area Assessment Guides
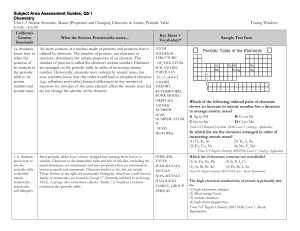
... A few other groups are given family names. These include the alkali metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The tr ...
... A few other groups are given family names. These include the alkali metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The tr ...
Ionization methods - 2-CI - Florida International University
... Occurs when an ion/atom or ion/molecule reaction takes place, in which the charge on the ion is transferred to the neutral species without dissociation of either. A++ B A + B+ Dissociative charge transfer Occurs when an ion/molecule reaction takes place, in which the charge on the ion is transferr ...
... Occurs when an ion/atom or ion/molecule reaction takes place, in which the charge on the ion is transferred to the neutral species without dissociation of either. A++ B A + B+ Dissociative charge transfer Occurs when an ion/molecule reaction takes place, in which the charge on the ion is transferr ...
Organic Chemistry Fifth Edition
... Alkyl halides are defined as primary if the carbon that the halogen is attached to is directly attached to one other carbon. Similarly if the carbon that the halogen is attached to is directly attached to two carbons then it is a secondary alkyl halide. In tertiary alkyl halides the carbon with the ...
... Alkyl halides are defined as primary if the carbon that the halogen is attached to is directly attached to one other carbon. Similarly if the carbon that the halogen is attached to is directly attached to two carbons then it is a secondary alkyl halide. In tertiary alkyl halides the carbon with the ...
Microwave-Assisted Sulfamide Synthesis
... unsymmetrical compounds rely on low-yielding synthetic steps that are neither general nor selective [1,5]. A novel transition-metal-catalyzed process for making unsymmetric sulfamides that was recently reported has several limitations, especially with ortho-isomers [1]. Even though other available m ...
... unsymmetrical compounds rely on low-yielding synthetic steps that are neither general nor selective [1,5]. A novel transition-metal-catalyzed process for making unsymmetric sulfamides that was recently reported has several limitations, especially with ortho-isomers [1]. Even though other available m ...
MLCT excited states and charge delocalization in some rutheniumÁ
... and the acceptor are very strongly mixed can exhibit unique properties that do differ markedly from those of the separated donor and acceptor. In principle, the degree to which these modifications in properties are important can be correlated to the amount of electronic charge delocalized between th ...
... and the acceptor are very strongly mixed can exhibit unique properties that do differ markedly from those of the separated donor and acceptor. In principle, the degree to which these modifications in properties are important can be correlated to the amount of electronic charge delocalized between th ...
acidic site
... We can increase the strength of an acid by adding electronwithdrawing groups, further stabilizing its conjugate base. e.g. To increase the acidity of acetic acid, replace one or more hydrogen atoms of the methyl group with halogens: ...
... We can increase the strength of an acid by adding electronwithdrawing groups, further stabilizing its conjugate base. e.g. To increase the acidity of acetic acid, replace one or more hydrogen atoms of the methyl group with halogens: ...
Chapter 6 Thermochemistry
... reactions that release heat are called exothermic reactions when DH is +, heat is being absorbed by the system reactions that release heat are called endothermic reactions chemical heat packs contain iron filings that are oxidized in an exothermic reaction ─ your hands get warm because the released ...
... reactions that release heat are called exothermic reactions when DH is +, heat is being absorbed by the system reactions that release heat are called endothermic reactions chemical heat packs contain iron filings that are oxidized in an exothermic reaction ─ your hands get warm because the released ...
Organic Chemistry Introduction
... • Solvents that can donate hydrogen bonds (-OH or –NH) slow SN2 reactions by associating with reactants • Energy is required to break interactions between reactant and solvent • Polar aprotic solvents (no NH, OH, SH) form weaker interactions with substrate and permit faster reaction ...
... • Solvents that can donate hydrogen bonds (-OH or –NH) slow SN2 reactions by associating with reactants • Energy is required to break interactions between reactant and solvent • Polar aprotic solvents (no NH, OH, SH) form weaker interactions with substrate and permit faster reaction ...