AS/A level
... Further evidence for this model comes from successive ionisation energies. Explain how these provide evidence for aspects of the model described. Sketch the expected pattern of successive ionisation energies for an atom of aluminium and use it to illustrate your answer. ...
... Further evidence for this model comes from successive ionisation energies. Explain how these provide evidence for aspects of the model described. Sketch the expected pattern of successive ionisation energies for an atom of aluminium and use it to illustrate your answer. ...
Solubility Workbook
... The following workbook is designed to ensure that you can demonstrate your understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of t ...
... The following workbook is designed to ensure that you can demonstrate your understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of t ...
Section 1.3 - The Student Room
... 2Fe + 3Cl2 Æ 2FeCl3 CaO + 2HNO3 Æ Ca(NO3)2 + H2O CaCO3 + 2HCl Æ CaCl2 + CO2 + H2O H2SO4 + 2NaOH Æ Na2SO4 + 2H2O 2HCl + Ca(OH)2 Æ CaCl2 + 2H2O 2Na + 2H2O Æ 2NaOH + H2 CH4 + 2O2 Æ CO2 + 2H2O 2CH3OH + 3O2 Æ 2CO2 + 4H2O ...
... 2Fe + 3Cl2 Æ 2FeCl3 CaO + 2HNO3 Æ Ca(NO3)2 + H2O CaCO3 + 2HCl Æ CaCl2 + CO2 + H2O H2SO4 + 2NaOH Æ Na2SO4 + 2H2O 2HCl + Ca(OH)2 Æ CaCl2 + 2H2O 2Na + 2H2O Æ 2NaOH + H2 CH4 + 2O2 Æ CO2 + 2H2O 2CH3OH + 3O2 Æ 2CO2 + 4H2O ...
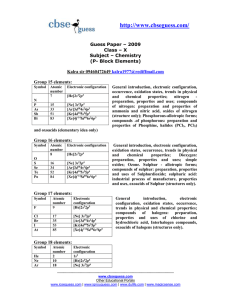
Amines(NCERT) CHEMISTRY TUTORIAL, III – A 41 Nehru Nagar
... is as follows: C6H5NH2< C6H5NHCH3< C6H5CH2NH2. (iv) In the gas phase, there is no solvation effect. As a result, the basic strength mainlydepends upon the +I effect. The higher the +I effect, the stronger is the base. Also, the greater the number of alkyl groups, the higher is the +I effect. Therefo ...
... is as follows: C6H5NH2< C6H5NHCH3< C6H5CH2NH2. (iv) In the gas phase, there is no solvation effect. As a result, the basic strength mainlydepends upon the +I effect. The higher the +I effect, the stronger is the base. Also, the greater the number of alkyl groups, the higher is the +I effect. Therefo ...
enjoy chemistry
... (ii)Noble gases are mostly chemically inert. Their inertness to chemical reactivity is attributed to the following reasons: (i) The noble gases except helium (1s2) have completely filled ns2p66 electronic configuration in their valence shell. (ii) They have high ionisation enthalpy and more positive ...
... (ii)Noble gases are mostly chemically inert. Their inertness to chemical reactivity is attributed to the following reasons: (i) The noble gases except helium (1s2) have completely filled ns2p66 electronic configuration in their valence shell. (ii) They have high ionisation enthalpy and more positive ...
Synthesis, crystal structure and vibrational spectra
... graphite monochromator using MoKα radiation. The chemical crystal data, the parameters used for the X-ray diffraction data collection and the results of crystal structure determinations of NaLa(PO3)4 and AgLa(PO3)4 are listed in Table 1. The empirical absorption corrections were carried out using SC ...
... graphite monochromator using MoKα radiation. The chemical crystal data, the parameters used for the X-ray diffraction data collection and the results of crystal structure determinations of NaLa(PO3)4 and AgLa(PO3)4 are listed in Table 1. The empirical absorption corrections were carried out using SC ...
physical setting chemistry
... heated in the flame of a gas burner. A characteristic color of light is emitted by these ions in the flame when the electrons (1) gain energy as they return to lower energy levels (2) gain energy as they move to higher energy levels (3) emit energy as they return to lower energy levels (4) emit ener ...
... heated in the flame of a gas burner. A characteristic color of light is emitted by these ions in the flame when the electrons (1) gain energy as they return to lower energy levels (2) gain energy as they move to higher energy levels (3) emit energy as they return to lower energy levels (4) emit ener ...
A high-field solid-state Cl NMR and quantum chemical
... biological processes.1 As the study of the structural and binding environments of inorganic atoms within biochemical systems continues to progress, the need for improved models and methods of study grows. Chlorine is of significant biochemical relevance as chloride ion channels are carriers of electr ...
... biological processes.1 As the study of the structural and binding environments of inorganic atoms within biochemical systems continues to progress, the need for improved models and methods of study grows. Chlorine is of significant biochemical relevance as chloride ion channels are carriers of electr ...
Chemistry Exemplar Problems
... phase, a conscious effort has been made to discourage rote learning and to enhance comprehension. This is well in tune with the NPE-1986 and Learning Without Burden-1993 that recommend child centred system of education. The textbooks for Class XI were released in 2006 and for Class XII in 2007. Over ...
... phase, a conscious effort has been made to discourage rote learning and to enhance comprehension. This is well in tune with the NPE-1986 and Learning Without Burden-1993 that recommend child centred system of education. The textbooks for Class XI were released in 2006 and for Class XII in 2007. Over ...
Cliffs Notes
... Trademarks: Cliffs, CliffsNotes, CliffsAP, CliffsComplete, CliffsTestPrep, CliffsQuickReview, CliffsNote-a-Day, and all related logos and trade dress are registered trademarks or trademarks of Hungry Minds, Inc., in the United States and other countries. AP, APP, and Advanced Placement Program are r ...
... Trademarks: Cliffs, CliffsNotes, CliffsAP, CliffsComplete, CliffsTestPrep, CliffsQuickReview, CliffsNote-a-Day, and all related logos and trade dress are registered trademarks or trademarks of Hungry Minds, Inc., in the United States and other countries. AP, APP, and Advanced Placement Program are r ...
M - coercingmolecules
... Cu2S) by a multistep process. After an initial grinding, the first step is to “roast” the ore (heat it strongly with O2) to form Cu2O and SO2 2Cu2S(s) + 3O2(g) ...
... Cu2S) by a multistep process. After an initial grinding, the first step is to “roast” the ore (heat it strongly with O2) to form Cu2O and SO2 2Cu2S(s) + 3O2(g) ...
20.2 Oxidation Numbers
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
Major 01 - KFUPM Faculty List
... 2 mol SO2 reacts with 1 mol O2, thus 1.4047 mol SO2 use (1.4047/2) mol O2 = 0.7024 mol O2 So of the initial 3.125 mol O2, 0.7024 mol O2 are used and (3.125 - 0.7024) mol O2 = 2.4226 mol O2 are left over (in excess). This is 2.4226 mol x 32 g O2/mol = 77.5 g O2 are left over (choice A). 15. 1.00 mL ...
... 2 mol SO2 reacts with 1 mol O2, thus 1.4047 mol SO2 use (1.4047/2) mol O2 = 0.7024 mol O2 So of the initial 3.125 mol O2, 0.7024 mol O2 are used and (3.125 - 0.7024) mol O2 = 2.4226 mol O2 are left over (in excess). This is 2.4226 mol x 32 g O2/mol = 77.5 g O2 are left over (choice A). 15. 1.00 mL ...
Density Functional Study of Molecular Orbitals of Ferrocene and
... well because any invading electrons for another molecules will fill in to the LUMO, that is why comparing the energies of these orbitals create an idea of how reactive a molecule is important parametric properties of the molecules at the DFT/B3LYP levels in 6-31G (d) basis set has been calculated an ...
... well because any invading electrons for another molecules will fill in to the LUMO, that is why comparing the energies of these orbitals create an idea of how reactive a molecule is important parametric properties of the molecules at the DFT/B3LYP levels in 6-31G (d) basis set has been calculated an ...
ADSORPTION OF Cr(NH3)6 3+ AND Cr(en)3 3+ ON CLAY
... chromium adsorbed is almost doubled, from 5 ppm for the Cr(NH3)G3§ complex to 9 ppm for the Cr(en)33+ complex. These results are similar to those previously reported (Koppelman and Dillard, 1977) for the adsorption of other transition metal ions on chlorite, illite, and kaolinite. The quantity of me ...
... chromium adsorbed is almost doubled, from 5 ppm for the Cr(NH3)G3§ complex to 9 ppm for the Cr(en)33+ complex. These results are similar to those previously reported (Koppelman and Dillard, 1977) for the adsorption of other transition metal ions on chlorite, illite, and kaolinite. The quantity of me ...
+2 - h2ochem
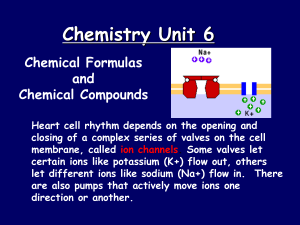
... closing of a complex series of valves on the cell membrane, called ion channels. Some valves let certain ions like potassium (K+) flow out, others let different ions like sodium (Na+) flow in. There are also pumps that actively move ions one direction or another. ...
... closing of a complex series of valves on the cell membrane, called ion channels. Some valves let certain ions like potassium (K+) flow out, others let different ions like sodium (Na+) flow in. There are also pumps that actively move ions one direction or another. ...
laman web smk raja perempuan, ipoh
... 14. explain the attractive forces between molecules (van der Waals forces and hydrogen bonding) l5. explain the meaning of Lewis acids and bases in terms of charge / electron density 16. explain why many organic compounds containing oxygen / nitrogen which have lone pair electrons (as Lewis bases) f ...
... 14. explain the attractive forces between molecules (van der Waals forces and hydrogen bonding) l5. explain the meaning of Lewis acids and bases in terms of charge / electron density 16. explain why many organic compounds containing oxygen / nitrogen which have lone pair electrons (as Lewis bases) f ...
Problem Authors - PianetaChimica
... The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl 2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Ola ...
... The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl 2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Ola ...
Topic 8 Acids and Bases File
... Acid and Base Worksheet 1) Using your knowledge of the Brønsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) HNO3 + OH- b) CH3NH2 + H2O c) OH- + HPO4-2 2) The compound NaOH is a base by all three of the th ...
... Acid and Base Worksheet 1) Using your knowledge of the Brønsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) HNO3 + OH- b) CH3NH2 + H2O c) OH- + HPO4-2 2) The compound NaOH is a base by all three of the th ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Electrolytes and Nonelectrolytes • in order to conduct electricity, a material must have charged particles that are able to flow • electrolyte solutions all contain ions dissolved in the water – ionic compounds are electrolytes because they all dissociate into their ions when they dissolve ...
... Electrolytes and Nonelectrolytes • in order to conduct electricity, a material must have charged particles that are able to flow • electrolyte solutions all contain ions dissolved in the water – ionic compounds are electrolytes because they all dissociate into their ions when they dissolve ...
ammines ncert solution
... In C6H5NH2, N is directly attached to the benzene ring. Thus, the lone pair of electrons on the N−atom is delocalized over the benzene ring. In C6H5CH2NH2, N is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the N ...
... In C6H5NH2, N is directly attached to the benzene ring. Thus, the lone pair of electrons on the N−atom is delocalized over the benzene ring. In C6H5CH2NH2, N is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the N ...
Studies Regarding the Nickel Electrodeposition from
... electroless Ag deposition onto Cu substrates from Ag ions containing choline chloride – ethylene glycol ionic liquids, which represents a technological procedure with a reduced impact on environment. The authors have shown that Ag deposition on Cu takes place due to the thermodynamic reduction of Ag ...
... electroless Ag deposition onto Cu substrates from Ag ions containing choline chloride – ethylene glycol ionic liquids, which represents a technological procedure with a reduced impact on environment. The authors have shown that Ag deposition on Cu takes place due to the thermodynamic reduction of Ag ...
LaBrake, Fundamentals Diagnostic Questions
... b) a homogenous mixture c) a mineral d) an element e) a compound 17. All of the following are considered subatomic particles, except: a) gamma rays (correct) b) electrons c) protons d) neutrons e) positrons 18. All of the following are statements from Dalton’s atomic hypothesis, except: a) All the a ...
... b) a homogenous mixture c) a mineral d) an element e) a compound 17. All of the following are considered subatomic particles, except: a) gamma rays (correct) b) electrons c) protons d) neutrons e) positrons 18. All of the following are statements from Dalton’s atomic hypothesis, except: a) All the a ...
PX312-1718
... 17. Which of Figures I–IV represent(s) the result of mixing aqueous solutions of Na2S and NiCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation, A = anion) ...
... 17. Which of Figures I–IV represent(s) the result of mixing aqueous solutions of Na2S and NiCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation, A = anion) ...
Rydberg-Stark deceleration of atoms and
... the production and confinement of anti-hydrogen [34]. In addition, confinement of Rb Rydberg atoms in optical lattices has also been achieved [35]. Applications of decelerated beams of Rydberg atoms and molecules Highly excited Rydberg states of atoms and molecules play important roles in many areas ...
... the production and confinement of anti-hydrogen [34]. In addition, confinement of Rb Rydberg atoms in optical lattices has also been achieved [35]. Applications of decelerated beams of Rydberg atoms and molecules Highly excited Rydberg states of atoms and molecules play important roles in many areas ...