Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents
... In the spectra of CS–Hg(II) and CSm–Hg(II), the interactions were mainly affirmed by the reduction of the intensity of the peak assigned to the –NH group in amine, confirming that nitrogen atoms are the main adsorption sites for Hg(II) adsorption on CS and CSm. It is well known that nitrogen–ligand ...
... In the spectra of CS–Hg(II) and CSm–Hg(II), the interactions were mainly affirmed by the reduction of the intensity of the peak assigned to the –NH group in amine, confirming that nitrogen atoms are the main adsorption sites for Hg(II) adsorption on CS and CSm. It is well known that nitrogen–ligand ...
Chapter 1 Introduction: Matter and Measurement
... • Na(11e) close to the noble gas Ne(10e), so Na is going to lose 1 e, Form Na+ ...
... • Na(11e) close to the noble gas Ne(10e), so Na is going to lose 1 e, Form Na+ ...
CHAPTER 16 ACID-BASE EQUILIBRIA AND SOLUBILITY
... pH = 4.44 Could you have predicted whether the pH should have increased or decreased after the addition of the sodium acetate to the pure 0.40 M acetic acid in part (a)? An alternate way to work part (b) of this problem is to use the Henderson-Hasselbalch equation. pH = pKa + log ...
... pH = 4.44 Could you have predicted whether the pH should have increased or decreased after the addition of the sodium acetate to the pure 0.40 M acetic acid in part (a)? An alternate way to work part (b) of this problem is to use the Henderson-Hasselbalch equation. pH = pKa + log ...
Chemistry Honours - SCS Autonomous College
... of Kapustinskii expression for lattice energy, Madelung constant, Born-Haber cycle and its application, Solvation energy (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach) Energetics of hybridization, equivalent and non-equivalent hybrid orbitals. Bent’s rule, Resonan ...
... of Kapustinskii expression for lattice energy, Madelung constant, Born-Haber cycle and its application, Solvation energy (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach) Energetics of hybridization, equivalent and non-equivalent hybrid orbitals. Bent’s rule, Resonan ...
Problem 1-2
... today’s air with the main component nitrogen. The main component A of the first atmosphere and the compounds ammonia and water react to form the second atmosphere with its gaseous main product B and two gaseous side products C and D: ...
... today’s air with the main component nitrogen. The main component A of the first atmosphere and the compounds ammonia and water react to form the second atmosphere with its gaseous main product B and two gaseous side products C and D: ...
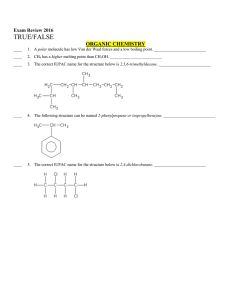
Multiple Choice Exam Review June 2016
... d. atoms which both have equally low ionization energies e. atoms which have low but unequal ionization energies According to VSEPR theory, molecules adjust their shapes to keep which of the following as far apart as possible? a. pairs of valence electrons d. mobile electrons b. inner shell electron ...
... d. atoms which both have equally low ionization energies e. atoms which have low but unequal ionization energies According to VSEPR theory, molecules adjust their shapes to keep which of the following as far apart as possible? a. pairs of valence electrons d. mobile electrons b. inner shell electron ...
Physical Sciences Grade 12 Term 2
... The very high frequency that ultrasound has a small wavelength. Therefore it can be ...
... The very high frequency that ultrasound has a small wavelength. Therefore it can be ...
Marks
... ALLOW –32.4 kJ OR –32400 J (Units must be shown) Award all 5 marks above for correct answer with no working IF 25 ºC has been used instead of 298 K, correctly calculated ∆G values are = –87 kJ mol–1 OR –87000 J mol–1 4 marks are still available up to this point and maximum possible from (e)(i) is 5 ...
... ALLOW –32.4 kJ OR –32400 J (Units must be shown) Award all 5 marks above for correct answer with no working IF 25 ºC has been used instead of 298 K, correctly calculated ∆G values are = –87 kJ mol–1 OR –87000 J mol–1 4 marks are still available up to this point and maximum possible from (e)(i) is 5 ...
Unit F325 - Equilibria, energetics and elements
... ALLOW –32.4 kJ OR –32400 J (Units must be shown) Award all 5 marks above for correct answer with no working IF 25 ºC has been used instead of 298 K, correctly calculated ∆G values are = –87 kJ mol–1 OR –87000 J mol–1 4 marks are still available up to this point and maximum possible from (e)(i) is 5 ...
... ALLOW –32.4 kJ OR –32400 J (Units must be shown) Award all 5 marks above for correct answer with no working IF 25 ºC has been used instead of 298 K, correctly calculated ∆G values are = –87 kJ mol–1 OR –87000 J mol–1 4 marks are still available up to this point and maximum possible from (e)(i) is 5 ...
Support Material
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
1049-1060 Reeder.pm - Mineralogical Society of America
... it is not commonly found as a major constituent. For example, Sr substitutes readily in the Ca site of calcite, as demonstrated by a Sr K-edge XAFS study of selected Sr-rich calcite samples (Pingitore et al. 1992). Heterovalent ions such as U4+ may also substitute in the Ca site as was recently show ...
... it is not commonly found as a major constituent. For example, Sr substitutes readily in the Ca site of calcite, as demonstrated by a Sr K-edge XAFS study of selected Sr-rich calcite samples (Pingitore et al. 1992). Heterovalent ions such as U4+ may also substitute in the Ca site as was recently show ...
Dynamic Equilibria in Solvent-Mediated Anion, Cation and Ligand
... evidence is given for such a mechanism or assignment. Compared to typical molecular complexes of low to medium molecular weights, many coordination polymers have a perceived low solubility in most conventional solvents and so are often described as “insoluble”.[17, 18, 29] In addition, it has been ...
... evidence is given for such a mechanism or assignment. Compared to typical molecular complexes of low to medium molecular weights, many coordination polymers have a perceived low solubility in most conventional solvents and so are often described as “insoluble”.[17, 18, 29] In addition, it has been ...
AS Chemistry Teacher Handbook
... Candidates should know that 12C is used as the standard in comparing relative masses. Candidates should be able to use relative atomic masses to calculate relative formula masses. Candidates will not be expected to draw a diagram of ...
... Candidates should know that 12C is used as the standard in comparing relative masses. Candidates should be able to use relative atomic masses to calculate relative formula masses. Candidates will not be expected to draw a diagram of ...
The Determination of the Location of Contact Electrification-Induced Discharge Events
... Contact electrificationsthe transfer of charge between two objects when they are brought into contact and then separatedsis ubiquitous;1,2 even two pieces of identical material brought into contact can result in charge separation.3-9 The phenomenon of contact electrification has been known for thous ...
... Contact electrificationsthe transfer of charge between two objects when they are brought into contact and then separatedsis ubiquitous;1,2 even two pieces of identical material brought into contact can result in charge separation.3-9 The phenomenon of contact electrification has been known for thous ...
Chemsheets AS 1027
... a) reaction of hydrochloric acid (aq) with potassium hydroxide (aq) b) precipitation of silver iodide from reaction between silver nitrate (aq) and potassium iodide (aq) c) reaction of potassium carbonate (aq) with nitric acid (aq) d) precipitation of calcium hydroxide from reaction between sodium h ...
... a) reaction of hydrochloric acid (aq) with potassium hydroxide (aq) b) precipitation of silver iodide from reaction between silver nitrate (aq) and potassium iodide (aq) c) reaction of potassium carbonate (aq) with nitric acid (aq) d) precipitation of calcium hydroxide from reaction between sodium h ...
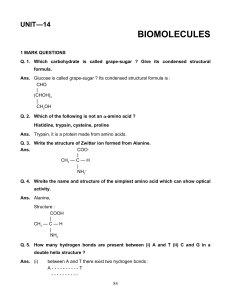
HOTS Worksheet
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
2 - equations
... a) reaction of hydrochloric acid (aq) with potassium hydroxide (aq) b) precipitation of silver iodide from reaction between silver nitrate (aq) and potassium iodide (aq) c) reaction of potassium carbonate (aq) with nitric acid (aq) d) precipitation of calcium hydroxide from reaction between sodium h ...
... a) reaction of hydrochloric acid (aq) with potassium hydroxide (aq) b) precipitation of silver iodide from reaction between silver nitrate (aq) and potassium iodide (aq) c) reaction of potassium carbonate (aq) with nitric acid (aq) d) precipitation of calcium hydroxide from reaction between sodium h ...
Schaum`s Outline of Theory and Problems of
... You must practice by working many problems, because in addition to the principles, you must get accustomed to the many details involved in solving problems correctly. The key to success in chemistry is working very many problems! To get the most from this book, use a 5 × 8 card to cover up the solut ...
... You must practice by working many problems, because in addition to the principles, you must get accustomed to the many details involved in solving problems correctly. The key to success in chemistry is working very many problems! To get the most from this book, use a 5 × 8 card to cover up the solut ...
A New Synthetic Method for Nanoscale Metal-
... resulting from their small size. Chapter 3 describes the validation of the Spray-Drying (SD) technique as a new methodology to synthesise NMOFs and their related hollow superstructures. The impact of the main experimental parameters on the synthesis of NMOFs is given as well as the different modes o ...
... resulting from their small size. Chapter 3 describes the validation of the Spray-Drying (SD) technique as a new methodology to synthesise NMOFs and their related hollow superstructures. The impact of the main experimental parameters on the synthesis of NMOFs is given as well as the different modes o ...
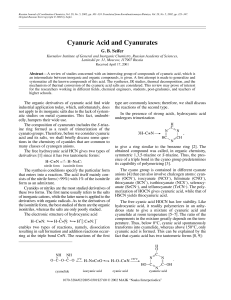
Cyanuric Acid and Cyanurates
... The sodium salt of cyanurtricyanamide (C3N3)(CN2H)3 was synthesized in [43]. This compound contains the (C3N3)(CN2)3– anion, which evidently can be used as the binding unit in the production of various polymers. The reaction of C3N3Cl3 with HI gives an amorphous brown compound (CNI)x [8] that decomp ...
... The sodium salt of cyanurtricyanamide (C3N3)(CN2H)3 was synthesized in [43]. This compound contains the (C3N3)(CN2)3– anion, which evidently can be used as the binding unit in the production of various polymers. The reaction of C3N3Cl3 with HI gives an amorphous brown compound (CNI)x [8] that decomp ...
Fundamentals of Environmental Chemistry
... reader knows nothing of the meaning of these terms. Chapter 2 discusses matter largely on the basis of its physical nature and behavior, introducing physical and chemical properties, states of matter, the mole as a quantity of matter, and other ideas required to visualize chemical substances as phys ...
... reader knows nothing of the meaning of these terms. Chapter 2 discusses matter largely on the basis of its physical nature and behavior, introducing physical and chemical properties, states of matter, the mole as a quantity of matter, and other ideas required to visualize chemical substances as phys ...
IIT-JEE - Brilliant Public School Sitamarhi
... Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of lattice vacancy as a r ...
... Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of lattice vacancy as a r ...
Effects of Copper and Zinc Ions on Photosystem II Studied by EPR
... monitored by the Cu2+ EPR signal. A 200 K illumination of such a sample revealed a slightly altered EPR spectrum. The yield of signals observed increased to ∼1.2 spins/PS II, and there was a very slight shift in the g-value to a higher value. After a second EDTA wash, 200 K illumination induced 2 ra ...
... monitored by the Cu2+ EPR signal. A 200 K illumination of such a sample revealed a slightly altered EPR spectrum. The yield of signals observed increased to ∼1.2 spins/PS II, and there was a very slight shift in the g-value to a higher value. After a second EDTA wash, 200 K illumination induced 2 ra ...
equilibrium - eVirtualGuru
... reactions also attain a state of equilibrium. These reactions can occur both in forward and backward directions. When the rates of the forward and reverse reactions become equal, the concentrations of the reactants and the products remain constant. This is the stage of chemical equilibrium. This equ ...
... reactions also attain a state of equilibrium. These reactions can occur both in forward and backward directions. When the rates of the forward and reverse reactions become equal, the concentrations of the reactants and the products remain constant. This is the stage of chemical equilibrium. This equ ...
Chemical Reactions - 2012 Book Archive
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...