PC_Chemistry_Macomb_April08
... The integrity of the scientific process depends on scientists and citizens understanding and respecting the “Nature of Science.” Openness to new ideas, skepticism, and honesty are attributes required for good scientific practice. Scientists must use logical reasoning during investigation design, ana ...
... The integrity of the scientific process depends on scientists and citizens understanding and respecting the “Nature of Science.” Openness to new ideas, skepticism, and honesty are attributes required for good scientific practice. Scientists must use logical reasoning during investigation design, ana ...
5 Steps
... Welcome to the AP Chemistry Five-Step Program. The fact that you are reading this preface suggests that you will be taking the AP exam in chemistry. The AP Chemistry exam is constantly evolving and so this guide has evolved. In this edition, we have updated the book to match the new AP Chemistry exa ...
... Welcome to the AP Chemistry Five-Step Program. The fact that you are reading this preface suggests that you will be taking the AP exam in chemistry. The AP Chemistry exam is constantly evolving and so this guide has evolved. In this edition, we have updated the book to match the new AP Chemistry exa ...
Study Guide for Content Mastery - Student Edition
... has six study guide pages of questions and exercises for you to complete as you read the text. The study guide pages are divided into sections that match those in your text. You will find that the directions in the Study Guide for Content Mastery are simply stated and easy to follow. Sometimes you w ...
... has six study guide pages of questions and exercises for you to complete as you read the text. The study guide pages are divided into sections that match those in your text. You will find that the directions in the Study Guide for Content Mastery are simply stated and easy to follow. Sometimes you w ...
5 Steps to a 5 AP Chemistry, 2008-2009 Edition
... constantly evolving and so this guide has evolved. In this edition, we have updated the book to match the new AP Chemistry exam, especially the changes in the free-response section. In the new exam, questions about laboratory experiments will be treated differently than in previous years. We have re ...
... constantly evolving and so this guide has evolved. In this edition, we have updated the book to match the new AP Chemistry exam, especially the changes in the free-response section. In the new exam, questions about laboratory experiments will be treated differently than in previous years. We have re ...
Tro Chemistry a Molecular Approach, 3E
... Let us carry this analogy one step further. Suppose we go on to cook our pizzas and accidentally burn one of them. Even though we theoretically have enough ingredients for three pizzas, we end up with only two. If this were a chemical reaction, the two pizzas would be our actual yield, the amount of ...
... Let us carry this analogy one step further. Suppose we go on to cook our pizzas and accidentally burn one of them. Even though we theoretically have enough ingredients for three pizzas, we end up with only two. If this were a chemical reaction, the two pizzas would be our actual yield, the amount of ...
Module 2
... change. If they do not come on time it will be counted as an absence. Long hair should be securely tied back to avoid the risk of setting it on fire. If large amounts of chemicals are spilled on your body, immediately remove the contaminated clothing and use the safety shower if available. Make sure ...
... change. If they do not come on time it will be counted as an absence. Long hair should be securely tied back to avoid the risk of setting it on fire. If large amounts of chemicals are spilled on your body, immediately remove the contaminated clothing and use the safety shower if available. Make sure ...
Specification – AS/A Level Chemistry A
... These specifications have been developed for students who wish to continue with a study of chemistry at Level 3 in the National Qualifications Framework (NQF). The AS specification has been written to provide progression from GCSE Science and GCSE Additional Science, or from GCSE Chemistry; achievem ...
... These specifications have been developed for students who wish to continue with a study of chemistry at Level 3 in the National Qualifications Framework (NQF). The AS specification has been written to provide progression from GCSE Science and GCSE Additional Science, or from GCSE Chemistry; achievem ...
IB Chemistry Online SAQ_Ans
... c From the equation, amount of H2SO4 = amount of NaOH ÷ 2 = 0.0125 mol in 20.0 cm3, so ‘scaling up’ to 1000 cm3 to obtain the concentration of diluted sulfuric acid 1000 × 0.0125mol dm −3 ...
... c From the equation, amount of H2SO4 = amount of NaOH ÷ 2 = 0.0125 mol in 20.0 cm3, so ‘scaling up’ to 1000 cm3 to obtain the concentration of diluted sulfuric acid 1000 × 0.0125mol dm −3 ...
9278654 PS/Chemistry Ja03 - Dolgeville Central School
... heated in the flame of a gas burner. A characteristic color of light is emitted by these ions in the flame when the electrons (1) gain energy as they return to lower energy levels (2) gain energy as they move to higher energy levels (3) emit energy as they return to lower energy levels (4) emit ener ...
... heated in the flame of a gas burner. A characteristic color of light is emitted by these ions in the flame when the electrons (1) gain energy as they return to lower energy levels (2) gain energy as they move to higher energy levels (3) emit energy as they return to lower energy levels (4) emit ener ...
Decrease = stress More Fe(OH) 2 dissolves in response Solubility
... – Separation of metals from their ores often involves dissolution – Qualitative analysis, i.e. identification of chemical species in solution, involves characteristic precipitation and dissolution reactions of salts – Water treatment/purification often involves precipitation of metals as insoluble i ...
... – Separation of metals from their ores often involves dissolution – Qualitative analysis, i.e. identification of chemical species in solution, involves characteristic precipitation and dissolution reactions of salts – Water treatment/purification often involves precipitation of metals as insoluble i ...
6 Chemical Bonding – Orbital Theory
... terms of the orbital theory of atomic structure. Heitler and London believed that electron cloud of the valence orbital on one atom ‘overlaps’ the electron cloud of the other bonding atom to form a covalent linkage. On the contrary, the electrovalent bond formation involves a physical transfer of th ...
... terms of the orbital theory of atomic structure. Heitler and London believed that electron cloud of the valence orbital on one atom ‘overlaps’ the electron cloud of the other bonding atom to form a covalent linkage. On the contrary, the electrovalent bond formation involves a physical transfer of th ...
U6B _13-14
... Molecular Equation: the typical equation you are use to writing keeping all molecules together Complete Ionic Equation: shows all the particles in a solution as they really exist, as IONS or MOLECULES. Anything aqueous needs to be split apart into the cation and anion Anything solid stays intact ...
... Molecular Equation: the typical equation you are use to writing keeping all molecules together Complete Ionic Equation: shows all the particles in a solution as they really exist, as IONS or MOLECULES. Anything aqueous needs to be split apart into the cation and anion Anything solid stays intact ...
Alkyl Chain Length Dependence of the Dynamics and Structure in
... beam from the pulse shaper, which generated pulses 1 and 2 in the 2D IR experiments and the single pump pulse in the PSPP experiments, was crossed in the sample with the probe pulse. The probe pulse, which carried the signal in pump−probe and vibrational echo experiments, was sent to a spectrometer ...
... beam from the pulse shaper, which generated pulses 1 and 2 in the 2D IR experiments and the single pump pulse in the PSPP experiments, was crossed in the sample with the probe pulse. The probe pulse, which carried the signal in pump−probe and vibrational echo experiments, was sent to a spectrometer ...
Now! - Soojeede.com
... Aspirin is acetylsalicylic acid while vitamin C is ascorbic acid; both are acids that can produce H ions when ionizing in water. Acetic acid (HC2H3O2) is a component of vinegar, hydrochloric acid (HCl) is stomach acid, phosphoric acid (H3PO4) is commonly found in dark soda pop, sulfuric acid (H2SO4) ...
... Aspirin is acetylsalicylic acid while vitamin C is ascorbic acid; both are acids that can produce H ions when ionizing in water. Acetic acid (HC2H3O2) is a component of vinegar, hydrochloric acid (HCl) is stomach acid, phosphoric acid (H3PO4) is commonly found in dark soda pop, sulfuric acid (H2SO4) ...
Name:
... changes only slightly and therefore does not offset the increase in size due to the increase in energy levels. Atomic radius decreases as you go left to right across a period in the periodic table. The valence electrons are found in orbitals of the same energy level. At the same time, the effective ...
... changes only slightly and therefore does not offset the increase in size due to the increase in energy levels. Atomic radius decreases as you go left to right across a period in the periodic table. The valence electrons are found in orbitals of the same energy level. At the same time, the effective ...
CO2 Dissociation using the Versatile Atmospheric Dielectric Barrier
... and pumping the CO2 gas into its final location puts a large limitation on the efficacy of carbon sequestration [8]. Even so, large amounts of research have and are currently being done to overcome these issues. ...
... and pumping the CO2 gas into its final location puts a large limitation on the efficacy of carbon sequestration [8]. Even so, large amounts of research have and are currently being done to overcome these issues. ...
Electrolyte Solutions: Thermodynamics, Crystallization
... Phase equilibria with systems containing electrolytes are of great importance. A few examples may illustrate this: Production of fertilizers and salts is often performed by precipitation of pure solids from multi component ionic solutions. Scaling in heat exchangers is caused by some salts for which ...
... Phase equilibria with systems containing electrolytes are of great importance. A few examples may illustrate this: Production of fertilizers and salts is often performed by precipitation of pure solids from multi component ionic solutions. Scaling in heat exchangers is caused by some salts for which ...
Ni recovery using KOH, NaOH, and NH4OH in the presence of
... heavy metals from the precipitated iron. EDTA has found several industrial applications as a chelating agent due to its high efficiency of metal extraction and the high thermodynamic stabilities of the metal complexes (Fisher et al., 1998), and because it has only minor impact on the physical and ch ...
... heavy metals from the precipitated iron. EDTA has found several industrial applications as a chelating agent due to its high efficiency of metal extraction and the high thermodynamic stabilities of the metal complexes (Fisher et al., 1998), and because it has only minor impact on the physical and ch ...
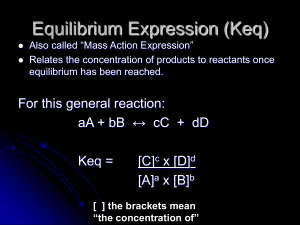
Equilibrium Expression (Keq)
... (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
... (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
4Chemical Quantities and Aqueous Reactions
... chemical reaction, the tomato sauce would be the limiting reactant, the reactant that limits the amount of product in a chemical reaction. Notice that the limiting reactant is simply the reactant that makes the least amount of product. Reactants that do not limit the amount of product—such as the cr ...
... chemical reaction, the tomato sauce would be the limiting reactant, the reactant that limits the amount of product in a chemical reaction. Notice that the limiting reactant is simply the reactant that makes the least amount of product. Reactants that do not limit the amount of product—such as the cr ...
IONIC EQULIBRIUM
... Electrolyte. A compound which produces positive and negative ions in solution. Strong electrolytes are completely dissociated, whereas weak electrolytes are only partially dissociated. Hydrolysis. An acid-base reaction of a cation or anion with water. Isoelectric point. The pH at which there is an e ...
... Electrolyte. A compound which produces positive and negative ions in solution. Strong electrolytes are completely dissociated, whereas weak electrolytes are only partially dissociated. Hydrolysis. An acid-base reaction of a cation or anion with water. Isoelectric point. The pH at which there is an e ...
Study materials of Chemistry for class XII
... Q10.Noble gases and metals crystallise with closed packed structures, yet the meeting points of noble gas crystals are exceptionally low. Why? 1M Ans. Noble gases crystallise in close packed structures, but the forces of interaction between the atoms are weak dispersion forces and they therefore hav ...
... Q10.Noble gases and metals crystallise with closed packed structures, yet the meeting points of noble gas crystals are exceptionally low. Why? 1M Ans. Noble gases crystallise in close packed structures, but the forces of interaction between the atoms are weak dispersion forces and they therefore hav ...
tailoringoxidepro
... to accommodate the extra charge30 . Film thickness also affects the metal-oxide interface adhesion, which can be further modified by suitable transition metal dopants31 . An alternative approach to modify the properties of oxides is to introduce dopant atoms into a host oxide. This can be achieved b ...
... to accommodate the extra charge30 . Film thickness also affects the metal-oxide interface adhesion, which can be further modified by suitable transition metal dopants31 . An alternative approach to modify the properties of oxides is to introduce dopant atoms into a host oxide. This can be achieved b ...
Document
... atom or ion that is bonded to more than one atom or molecule. Some of the most interesting ions have a metal ion surrounded by a number of ligands. Ligands are molecules, such as ammonia, NH3, or anions, such as cyanide, CN −, that readily bond to metal ions. Figure 5 shows a model of one complex io ...
... atom or ion that is bonded to more than one atom or molecule. Some of the most interesting ions have a metal ion surrounded by a number of ligands. Ligands are molecules, such as ammonia, NH3, or anions, such as cyanide, CN −, that readily bond to metal ions. Figure 5 shows a model of one complex io ...
Hybridization of atomic orbitals
... equivalent. The CH4 molecule is the most cited molecule to have a tetrahedral shape. Other molecules and ions having tetrahedral shapes are SiO44-, SO42-, As are the cases with sp2, hybrid orbitals, one or two of the sp3 hybrid orbitals may be occupied by non-bonding electrons. Water and ammonia are ...
... equivalent. The CH4 molecule is the most cited molecule to have a tetrahedral shape. Other molecules and ions having tetrahedral shapes are SiO44-, SO42-, As are the cases with sp2, hybrid orbitals, one or two of the sp3 hybrid orbitals may be occupied by non-bonding electrons. Water and ammonia are ...