AP Chemistry
... 1415. Molecular solids (A) melt at lower temperatures than ionic solids (B) cannot sublime (C) contain at least one hydrogen bond (D) always contain multiple covalent bonds (E) are packed tightly into a crystal lattice 1416. An example of a molecular compound that exists as a solid at STP is (D) C3H ...
... 1415. Molecular solids (A) melt at lower temperatures than ionic solids (B) cannot sublime (C) contain at least one hydrogen bond (D) always contain multiple covalent bonds (E) are packed tightly into a crystal lattice 1416. An example of a molecular compound that exists as a solid at STP is (D) C3H ...
+ OH - (aq) - Miss Gerges
... 28.0 mL of 0.250M HNO3 and 53.0 mL of 0.320M KOH are mixed. Calculate the amount of water formed in the resulting reaction. What are the concentrations of H+ and OH- ions in excess after the reaction goes to completion? Net ionic equation: H+ (aq) + OH- (aq) H2O (l) From volume and conc. find th ...
... 28.0 mL of 0.250M HNO3 and 53.0 mL of 0.320M KOH are mixed. Calculate the amount of water formed in the resulting reaction. What are the concentrations of H+ and OH- ions in excess after the reaction goes to completion? Net ionic equation: H+ (aq) + OH- (aq) H2O (l) From volume and conc. find th ...
chapter 5 - chemical reactions
... Reactions involving ionic compounds or acids and bases that occur in aqueous solutions can be represented by three different types of equations: the molecular equation, total ionic equation, and net ionic equation. Molecular equation - equation written with each compound represented by its formula; ...
... Reactions involving ionic compounds or acids and bases that occur in aqueous solutions can be represented by three different types of equations: the molecular equation, total ionic equation, and net ionic equation. Molecular equation - equation written with each compound represented by its formula; ...
3.98 MB - KFUPM Resources v3
... 28.0 mL of 0.250M HNO3 and 53.0 mL of 0.320M KOH are mixed. Calculate the amount of water formed in the resulting reaction. What are the concentrations of H+ and OH- ions in excess after the reaction goes to completion? Net ionic equation: H+ (aq) + OH- (aq) H2O (l) From volume and conc. find th ...
... 28.0 mL of 0.250M HNO3 and 53.0 mL of 0.320M KOH are mixed. Calculate the amount of water formed in the resulting reaction. What are the concentrations of H+ and OH- ions in excess after the reaction goes to completion? Net ionic equation: H+ (aq) + OH- (aq) H2O (l) From volume and conc. find th ...
Appendix N CONCENTRATION UNITS
... Chemical equations may take many forms. The statement above that iron oxide reacts with charcoal to produce elemental iron and carbon dioxide is one form of describing a chemical reaction. However, this type of statement does not allow any quantitative information to be determined. Using the informa ...
... Chemical equations may take many forms. The statement above that iron oxide reacts with charcoal to produce elemental iron and carbon dioxide is one form of describing a chemical reaction. However, this type of statement does not allow any quantitative information to be determined. Using the informa ...
Naming Compounds - Kowenscience.com
... • Chromium(IV) oxide. Cr is the symbol for chromium. O is the symbol for oxygen, but • take the first part of the element name (the root) and add –ide to get the name oxide. • Since chromium can have more than one charge, a Roman numeral must be used to identify that charge. • There are two oxygen i ...
... • Chromium(IV) oxide. Cr is the symbol for chromium. O is the symbol for oxygen, but • take the first part of the element name (the root) and add –ide to get the name oxide. • Since chromium can have more than one charge, a Roman numeral must be used to identify that charge. • There are two oxygen i ...
chemistry module p
... Chlorine is listed with an atomic mass of 35.45. All Chlorine atoms contain 17 protons in their nucleus. From the atomic mass above it could be concluded that a chlorine nucleus contains 18.45 neutrons; but there is no such thing as 0.45 of a neutron. An atomic mass, as appears on the periodic table ...
... Chlorine is listed with an atomic mass of 35.45. All Chlorine atoms contain 17 protons in their nucleus. From the atomic mass above it could be concluded that a chlorine nucleus contains 18.45 neutrons; but there is no such thing as 0.45 of a neutron. An atomic mass, as appears on the periodic table ...
View PDF - Oriental Journal of Chemistry
... for bonding, only 4 vectors are taken into account whereas if IR or Raman Spectroscopy is being considered, 3x5 =15 ( 3xN, N = number of atoms in the molecule) Cartesian coordinates will be considered. The use of 3N vectors is not only cumbersome but also the level of mathematical theory accompanyin ...
... for bonding, only 4 vectors are taken into account whereas if IR or Raman Spectroscopy is being considered, 3x5 =15 ( 3xN, N = number of atoms in the molecule) Cartesian coordinates will be considered. The use of 3N vectors is not only cumbersome but also the level of mathematical theory accompanyin ...
Chemistry II Exams and Keys Corrected 2016 Season
... you are taking on the scantron. 1. The density of a pure CH4 sample confined in a rigid container is 1.60 g/L at -73.0 oC. What would be the temperature in oC in the container, if the pressure is changed to 3.28 atm? A. 27.0ᵒC ...
... you are taking on the scantron. 1. The density of a pure CH4 sample confined in a rigid container is 1.60 g/L at -73.0 oC. What would be the temperature in oC in the container, if the pressure is changed to 3.28 atm? A. 27.0ᵒC ...
Astrochemistry and Star Formation
... assorted sources and predict abundances of molecules as functions of time and physical conditions. It is the modelers who are at the core of the field, since it is they who compare calculated and observational results to deduce details of the present and perhaps the past of the source. Chemical mode ...
... assorted sources and predict abundances of molecules as functions of time and physical conditions. It is the modelers who are at the core of the field, since it is they who compare calculated and observational results to deduce details of the present and perhaps the past of the source. Chemical mode ...
reactions taking place within cells
... Thermodynamics Study of conversion of energy between heat and other forms Thermochemistry Relationship between chemical reactions and heat changes Enthalpy H Measure of energy(heat content) Change Final value minus the initial value Enthalpy change H kJmol –1 Heat energy transferred in a reaction ...
... Thermodynamics Study of conversion of energy between heat and other forms Thermochemistry Relationship between chemical reactions and heat changes Enthalpy H Measure of energy(heat content) Change Final value minus the initial value Enthalpy change H kJmol –1 Heat energy transferred in a reaction ...
orange review book_2014_key
... 19. One similarity between all mixtures and compounds is that both (1) are heterogeneous (2) consist of two or more substances (3) are homogeneous (4) are heterogeneous 20. A dilute, aqueous potassium nitrate solution is best classified as a (1) homogeneous compound (2) homogeneous mixture ...
... 19. One similarity between all mixtures and compounds is that both (1) are heterogeneous (2) consist of two or more substances (3) are homogeneous (4) are heterogeneous 20. A dilute, aqueous potassium nitrate solution is best classified as a (1) homogeneous compound (2) homogeneous mixture ...
A millennial overview of transition metal chemistry
... interesting compounds are included in these categories, transition metal chemistry would finally have reached an impasse had X-ray crystallography not become a practical tool. Today, of course, the ready availability of structural information has revolutionized the transition element chemist’s modus ...
... interesting compounds are included in these categories, transition metal chemistry would finally have reached an impasse had X-ray crystallography not become a practical tool. Today, of course, the ready availability of structural information has revolutionized the transition element chemist’s modus ...
Aqueous Solutions
... nonmetals other than hydrogen – Nomenclature must include prefixes that specify the number of atoms of each element in the compound. • Use the minimum number of prefixes necessary to specify the compound. – Frequently drop the prefix mono- 1 ...
... nonmetals other than hydrogen – Nomenclature must include prefixes that specify the number of atoms of each element in the compound. • Use the minimum number of prefixes necessary to specify the compound. – Frequently drop the prefix mono- 1 ...
SUPPLEMENTAL PROBLEMS FOR CHEM 110
... The reaction is exothermic with ΔH = −46.2 kJ. The reaction is endothermic with ΔH = −92.4 kJ. The reaction is exothermic with ΔH = 92.4 kJ. The reaction is endothermic with ΔH = 92.4 kJ. The reaction is endothermic with ΔH = 46.2 kJ. ...
... The reaction is exothermic with ΔH = −46.2 kJ. The reaction is endothermic with ΔH = −92.4 kJ. The reaction is exothermic with ΔH = 92.4 kJ. The reaction is endothermic with ΔH = 92.4 kJ. The reaction is endothermic with ΔH = 46.2 kJ. ...
Oxidation-Reduction Reactions
... until the object is destroyed. One way to prevent corrosion is to protect the surface of the metal. Covering the surface of a metal object with paint or oil will prevent corrosion by sealing the metal off from any surrounding oxygen. Unfortunately, these protective coatings may eventually wear off o ...
... until the object is destroyed. One way to prevent corrosion is to protect the surface of the metal. Covering the surface of a metal object with paint or oil will prevent corrosion by sealing the metal off from any surrounding oxygen. Unfortunately, these protective coatings may eventually wear off o ...
315.pdf
... Kondo theory proposed by Cornut and single-impurity the broad maximum in resistivity, such as that Coqblin, observed in CePdSb at 150 K, is associated with the combined eff'ect of the crystalline electric fields on the localized 4f moments and Kondo-type interaction. This theory also predicts differ ...
... Kondo theory proposed by Cornut and single-impurity the broad maximum in resistivity, such as that Coqblin, observed in CePdSb at 150 K, is associated with the combined eff'ect of the crystalline electric fields on the localized 4f moments and Kondo-type interaction. This theory also predicts differ ...
Formulas, Reactions, Equations, and Moles
... • An Ionic compound can quickly be determined if a METAL (or cation) is bonded to a NONMETAL (or anion). • Naming Ionic compounds with metals that have only ONE oxidation state is fairly simple. ...
... • An Ionic compound can quickly be determined if a METAL (or cation) is bonded to a NONMETAL (or anion). • Naming Ionic compounds with metals that have only ONE oxidation state is fairly simple. ...
Force-field dependence of the conformational properties of α,ω
... solute-solvent contacts. The energy minimisations were terminated when the energy change per step became smaller than 0.1 kJ mol−1 . For the non-bonded interactions, a triple-range method with cutoff radii of 0.8/1.4 nm was used. Short-range van der Waals and electrostatic interactions were evaluate ...
... solute-solvent contacts. The energy minimisations were terminated when the energy change per step became smaller than 0.1 kJ mol−1 . For the non-bonded interactions, a triple-range method with cutoff radii of 0.8/1.4 nm was used. Short-range van der Waals and electrostatic interactions were evaluate ...
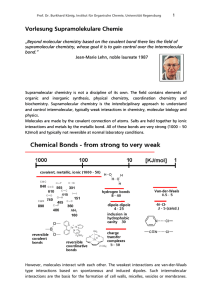
Vorlesung Supramolekulare Chemie
... energy. The eclipsed conformation is certainly higher in energy. TΔSconformation ~ RT ln 1/3 ~ 3 KJ/mol ...
... energy. The eclipsed conformation is certainly higher in energy. TΔSconformation ~ RT ln 1/3 ~ 3 KJ/mol ...
UV Spectroscopy
... - orbitals are the lowest energy occupied molecular orbitals * - orbitals are the highest energy unoccupied molecular orbitals - orbitals are of somewhat higher energy occupied molecular orbitals * - orbitals are lower in energy (unoccupied molecular orbitals) than * n - orbitals; Unshared pa ...
... - orbitals are the lowest energy occupied molecular orbitals * - orbitals are the highest energy unoccupied molecular orbitals - orbitals are of somewhat higher energy occupied molecular orbitals * - orbitals are lower in energy (unoccupied molecular orbitals) than * n - orbitals; Unshared pa ...
Density Functional Theory Based Study of the Electron Transfer
... The Journal of Physical Chemistry C ...
... The Journal of Physical Chemistry C ...
9701/04 - StudyGuide.PK
... (ii) Explain how the variation in conductivity is related to the structure and bonding in the elements. ...
... (ii) Explain how the variation in conductivity is related to the structure and bonding in the elements. ...