Chemistry
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus Content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus Content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
"Supramolecular chemistry is the chemistry of the intermolecular
... chemistry of tailor-shaped intermolecular interaction. In 'supramolecules' information is stored in the form of structural peculiarities. Moreover, not only the combined action of molecules is called supramolecular, but also the combined action of characteristic parts of one and the same molecule." ...
... chemistry of tailor-shaped intermolecular interaction. In 'supramolecules' information is stored in the form of structural peculiarities. Moreover, not only the combined action of molecules is called supramolecular, but also the combined action of characteristic parts of one and the same molecule." ...
Analytical Chemistry
... (concentration or composition) of various substances in samples. There are many methods of determining these amounts, all based on chemical or physical ...
... (concentration or composition) of various substances in samples. There are many methods of determining these amounts, all based on chemical or physical ...
Chemical Reactivity as Described by Quantum Chemical Methods
... (Other concepts introduced in this framework are reviewed in [39, 40]) In this way it is shown that DFT gave the possibility to sharply define concepts known for a long time in chemistry, but to which inadequate precision could be given to use them with confidence in quantitative studies. The last 1 ...
... (Other concepts introduced in this framework are reviewed in [39, 40]) In this way it is shown that DFT gave the possibility to sharply define concepts known for a long time in chemistry, but to which inadequate precision could be given to use them with confidence in quantitative studies. The last 1 ...
Oxidation-Reduction Reactions - An Introduction to Chemistry
... The oxidation of nitrogen to form nitrogen monoxide is very similar to the oxidation of zinc to form zinc oxide. N2(g) + O2(g) → 2NO(g) 2Zn(s) + O2(g) → 2ZnO(s) The main difference between these reactions is that as the nitrogen monoxide forms, electrons are not transferred completely, as occurs in ...
... The oxidation of nitrogen to form nitrogen monoxide is very similar to the oxidation of zinc to form zinc oxide. N2(g) + O2(g) → 2NO(g) 2Zn(s) + O2(g) → 2ZnO(s) The main difference between these reactions is that as the nitrogen monoxide forms, electrons are not transferred completely, as occurs in ...
IMPACT IONIZATION AND HIGH FIELD EFFECTS IN
... elds. In particular, the role of such e ects in the performance of submicron Si and GaAs devices has been uncertain at best. In recent years, interest has developed in wide-bandgap semiconductors such as ZnS, GaN and SiC for optoelectronic and high-power, high-frequency electronic applications. In ...
... elds. In particular, the role of such e ects in the performance of submicron Si and GaAs devices has been uncertain at best. In recent years, interest has developed in wide-bandgap semiconductors such as ZnS, GaN and SiC for optoelectronic and high-power, high-frequency electronic applications. In ...
Tests
... Chemistry is the study of the ________________ of matter and the __________ that matter undergoes. Matter is anything that has ___________ and takes up space. A _______________ would study the structure of the hemoglobin molecule and how it transports oxygen. An organic chemistry works mainly with _ ...
... Chemistry is the study of the ________________ of matter and the __________ that matter undergoes. Matter is anything that has ___________ and takes up space. A _______________ would study the structure of the hemoglobin molecule and how it transports oxygen. An organic chemistry works mainly with _ ...
THE KINETICS OF CHEMICAL REACTIONS: SINGLE
... the time, it will be close to Eq.3 although, occasionally, significant deviations may be observed. The noise will however go away if we repeat the same experiment with a much larger number of molecules. Even though each individual molecule is random, a large collection (or, as physicists call it, an ...
... the time, it will be close to Eq.3 although, occasionally, significant deviations may be observed. The noise will however go away if we repeat the same experiment with a much larger number of molecules. Even though each individual molecule is random, a large collection (or, as physicists call it, an ...
to view
... In the crystal of FeO, some of the Fe2+ cations are replaced by Fe3+ ions. Three Fe2+ ions are replaced by two Fe3+ ions to make up for the loss of positive charge. Thus there would be less amount of metal as compared to stoichiometric ...
... In the crystal of FeO, some of the Fe2+ cations are replaced by Fe3+ ions. Three Fe2+ ions are replaced by two Fe3+ ions to make up for the loss of positive charge. Thus there would be less amount of metal as compared to stoichiometric ...
M for Moles - Shop
... Schematic representation of an atom (in this case is a helium atom) A proton has a positive charge (+1) and an electron has a negative charge (-1). Neutron does not have a charge. It is neutral (0). All atoms have equal number of protons and electrons. In other words, they are always neutral. Charge ...
... Schematic representation of an atom (in this case is a helium atom) A proton has a positive charge (+1) and an electron has a negative charge (-1). Neutron does not have a charge. It is neutral (0). All atoms have equal number of protons and electrons. In other words, they are always neutral. Charge ...
PDF File
... Metal Ion Rescue and Functional Detection of Ribozyme Ligands of Specific Catalytic Metal Ions. As noted in the introduction, metal ion rescue experiments provide a powerful means to identify functional interactions and have been used extensively for protein and RNA enzymes. This approach has been e ...
... Metal Ion Rescue and Functional Detection of Ribozyme Ligands of Specific Catalytic Metal Ions. As noted in the introduction, metal ion rescue experiments provide a powerful means to identify functional interactions and have been used extensively for protein and RNA enzymes. This approach has been e ...
Atomic Structure
... For the energy levels in an atom, which one of the following statement is incorrect? (a) There are seven principle electron energy levels (b) The second principal energy level can have four sub-energy levels and contain a Maximum of eight electrons (c) The M energy level can have a maximum of 32 ele ...
... For the energy levels in an atom, which one of the following statement is incorrect? (a) There are seven principle electron energy levels (b) The second principal energy level can have four sub-energy levels and contain a Maximum of eight electrons (c) The M energy level can have a maximum of 32 ele ...
AP syllabus
... COURSE DESCRIPTION: The class meets 4 days each week for 70 minutes and one day each week for 90 minutes. Each week the 90 minute class will be devoted to laboratory work. Students keep a formal laboratory notebook. The notebook will be graded after each lab. Students should have completed courses i ...
... COURSE DESCRIPTION: The class meets 4 days each week for 70 minutes and one day each week for 90 minutes. Each week the 90 minute class will be devoted to laboratory work. Students keep a formal laboratory notebook. The notebook will be graded after each lab. Students should have completed courses i ...
GCSE Getting Started
... and neutrons, surrounded by electrons in shells *C1.3: Recall the relative charge and relative mass of: a) a proton b) a neutron c) an electron *C1.4: Explain why atoms contain equal numbers of protons and electrons *C1.5: Describe the nucleus of an atom as very small compared to the overall s ...
... and neutrons, surrounded by electrons in shells *C1.3: Recall the relative charge and relative mass of: a) a proton b) a neutron c) an electron *C1.4: Explain why atoms contain equal numbers of protons and electrons *C1.5: Describe the nucleus of an atom as very small compared to the overall s ...
Electrodeposition of rare earth metals in ionic liquids
... cationic blended ionic liquids which were constituted by the 2‐ hydroxyethyltrimethylammonium bis(trifluoromethylsulfonyl)imide Ch‐TFSI and P[225]‐TFSI was applied for the electro‐recovery of neodymium. In this study the starting materials were voice coil motors for hard disk drivers ...
... cationic blended ionic liquids which were constituted by the 2‐ hydroxyethyltrimethylammonium bis(trifluoromethylsulfonyl)imide Ch‐TFSI and P[225]‐TFSI was applied for the electro‐recovery of neodymium. In this study the starting materials were voice coil motors for hard disk drivers ...
Chapter 4
... current must flow from one electrode to the other, thus completing the circuit. Pure water is a very poor conductor of electricity. However, if we add a small amount of sodium chloride (NaCl), the bulb will glow as soon as the salt dissolves in the water. Solid NaCl, an ionic compound, breaks up int ...
... current must flow from one electrode to the other, thus completing the circuit. Pure water is a very poor conductor of electricity. However, if we add a small amount of sodium chloride (NaCl), the bulb will glow as soon as the salt dissolves in the water. Solid NaCl, an ionic compound, breaks up int ...
Reactions Balancing Chemical Equations uses Law of conservation
... What is oxidized? What is reduced? ...
... What is oxidized? What is reduced? ...
Side Chain Chemistry Mediates Backbone Fragmentation in
... There are a variety of potential scaffolds for delivering chemical functional groups to peptides via noncovalent interactions.14-17 Arguably, 18-crown-6 (18C6) is the preferred solution due to its ability to recognize protonated primary amines.18 Any lysine containing peptide is therefore an excelle ...
... There are a variety of potential scaffolds for delivering chemical functional groups to peptides via noncovalent interactions.14-17 Arguably, 18-crown-6 (18C6) is the preferred solution due to its ability to recognize protonated primary amines.18 Any lysine containing peptide is therefore an excelle ...
1. Atomic Structure and Periodic Table THE MASS SPECTROMETER
... in CaCl2 = -1 and not -2 because there are two Cl’s Always work out the oxidation for one atom of the element ...
... in CaCl2 = -1 and not -2 because there are two Cl’s Always work out the oxidation for one atom of the element ...
chm 205 - National Open University of Nigeria
... silicon and germanium are semi-metals; tin and lead are distinctly metallic in nature. Their common oxidation states are II and IV. In Unit 2 we discuss the salient features of the chemistry of nitrogen, phosphorus, arsenic, antimony and bismuth which constitute Group 15 of the periodic table. The m ...
... silicon and germanium are semi-metals; tin and lead are distinctly metallic in nature. Their common oxidation states are II and IV. In Unit 2 we discuss the salient features of the chemistry of nitrogen, phosphorus, arsenic, antimony and bismuth which constitute Group 15 of the periodic table. The m ...
UILChemistryProblemsPart2
... moving toward the left. If Q< Kc reaction is on the left moving toward the right. When a reaction starts with only reactants (no products), Q will increase as it approaches equilibrium. Q is a number that changes as the concentrations of the reactants and products change (as the reaction proceeds to ...
... moving toward the left. If Q< Kc reaction is on the left moving toward the right. When a reaction starts with only reactants (no products), Q will increase as it approaches equilibrium. Q is a number that changes as the concentrations of the reactants and products change (as the reaction proceeds to ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... 5. Properties: Clear or transparent but may be colored, Solute doesn’t settle on standing, Can’t be separated by filtering, Components are not in a definite ratio 6. Example: NaCl(aq) ...
... 5. Properties: Clear or transparent but may be colored, Solute doesn’t settle on standing, Can’t be separated by filtering, Components are not in a definite ratio 6. Example: NaCl(aq) ...
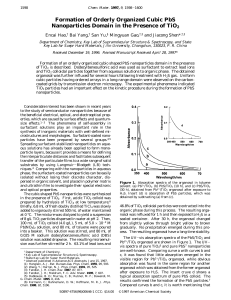
Formation of Orderly Organized Cubic PbS Nanoparticles Domain in
... organic phase during this process. The resulting organosol was refluxed for 1 h and then exposed to H2S in a sealed container. After 30 h, the organosol changed from slightly yellow through bright yellow to brown gradually. No precipitation emerged during this process. The resulting organosol have a ...
... organic phase during this process. The resulting organosol was refluxed for 1 h and then exposed to H2S in a sealed container. After 30 h, the organosol changed from slightly yellow through bright yellow to brown gradually. No precipitation emerged during this process. The resulting organosol have a ...
Practice Test Material - Directorate of Education
... Calculate the pH of 0.10M ammonia solution. Calculate the pH after 50.0 ml of this solution is treated with 25.0 ml of 0.10M HCl. The dissociation constant of ammonia (Kb) is 1.77×10–5. Hint – In the final condition, basic buffer is formed due to the presence of NH4Cl and NH4OH in the same solution. ...
... Calculate the pH of 0.10M ammonia solution. Calculate the pH after 50.0 ml of this solution is treated with 25.0 ml of 0.10M HCl. The dissociation constant of ammonia (Kb) is 1.77×10–5. Hint – In the final condition, basic buffer is formed due to the presence of NH4Cl and NH4OH in the same solution. ...