Ionic Conductivity in the Metal–Organic Framework UiO
... values of ionic conductivity, the clear difference in the activation energies of 0.18 and 0.35 eV for the grafted and deprotonated materials, respectively, reflects the contrast in local environment of the Li + ions in two materials. Both the higher activation energy and lower ionic conductivity are ...
... values of ionic conductivity, the clear difference in the activation energies of 0.18 and 0.35 eV for the grafted and deprotonated materials, respectively, reflects the contrast in local environment of the Li + ions in two materials. Both the higher activation energy and lower ionic conductivity are ...
Sructural and chemisorption properties of metallic surfaces and metallic overlayers
... For many years the study of the modification of surface structure has been an amazing topic th a t interests surface scientists. In order to extend the understanding of modified metallic surfaces in the field of electronic and atomic structure of adsorbate covered surfaces, two bimetallic systems (P ...
... For many years the study of the modification of surface structure has been an amazing topic th a t interests surface scientists. In order to extend the understanding of modified metallic surfaces in the field of electronic and atomic structure of adsorbate covered surfaces, two bimetallic systems (P ...
Ionic Liquids in Separation of Metal Ions from Aqueous
... extracted (D > 24) with imidazolium and pyridinium ionic liquids, while the other metal cations are not transferred to the IL phase. Moreover, good extraction abilities of imidazolium ILs with [NfO-] have been confirmed in the studies on Li+, Na+, Cs+, Ca2+, Sr2+ and La3+ extraction (Kozonoi & Ikeda ...
... extracted (D > 24) with imidazolium and pyridinium ionic liquids, while the other metal cations are not transferred to the IL phase. Moreover, good extraction abilities of imidazolium ILs with [NfO-] have been confirmed in the studies on Li+, Na+, Cs+, Ca2+, Sr2+ and La3+ extraction (Kozonoi & Ikeda ...
Worked out problems
... reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons on the product side of the equation), which occurs at the anode. The I– ions are the source of electrons, and t ...
... reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons on the product side of the equation), which occurs at the anode. The I– ions are the source of electrons, and t ...
Sample Exercise 20.1 Identifying Oxidizing and Reducing Agents
... reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons on the product side of the equation), which occurs at the anode. The I– ions are the source of electrons, and t ...
... reduction process (electrons on the reactant side of the equation). By definition, the reduction process occurs at the cathode. The second half-reaction is the oxidation process (electrons on the product side of the equation), which occurs at the anode. The I– ions are the source of electrons, and t ...
Physical chemistry and transition elements 5.1 Rates, equilibrium
... A d-block element is one in which electrons are filling d-orbitals and the highest energy sub-shell is a d-subshell. A transition element is a d-block element which forms at least one ion with an incomplete d-sub-shell. ...
... A d-block element is one in which electrons are filling d-orbitals and the highest energy sub-shell is a d-subshell. A transition element is a d-block element which forms at least one ion with an incomplete d-sub-shell. ...
〈541〉 TITRIMETRY
... be formed rapidly enough that the analysis time is practical. When the analytical reaction is not rapid, a residual titration may sometimes be successful. In general, complexometric indicators are themselves complexing agents. The reaction between metal ion and indicator must be rapid and reversible ...
... be formed rapidly enough that the analysis time is practical. When the analytical reaction is not rapid, a residual titration may sometimes be successful. In general, complexometric indicators are themselves complexing agents. The reaction between metal ion and indicator must be rapid and reversible ...
Photoprocesses in protoplanetary disks
... the 10 and 20 mm silicate Si–O stretching and O–Si–O bending mode features of two T Tauri stars, shifted by +0.4 and +0.2 for clarity. Bottom: normalized absorption efficiencies Qabs for models of spherical amorphous olivines with various sizes, calculated using the distribution of hollow spheres (DHS ...
... the 10 and 20 mm silicate Si–O stretching and O–Si–O bending mode features of two T Tauri stars, shifted by +0.4 and +0.2 for clarity. Bottom: normalized absorption efficiencies Qabs for models of spherical amorphous olivines with various sizes, calculated using the distribution of hollow spheres (DHS ...
chemistry (9189)
... bonding; covalent bonding; hydrogen bonding, other intermolecular interactions; metallic bonding) on the physical properties of substances ...
... bonding; covalent bonding; hydrogen bonding, other intermolecular interactions; metallic bonding) on the physical properties of substances ...
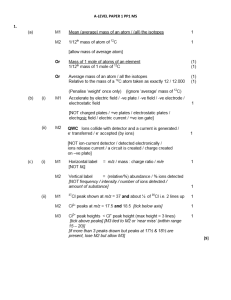
1 - A-Level Chemistry
... no lone / spare / non-bonded pair of electrons only score M2 if M1 correct or give ‘H’ in M1 ...
... no lone / spare / non-bonded pair of electrons only score M2 if M1 correct or give ‘H’ in M1 ...
The p-Block Elements The p-Block Elements
... to increase in size and metallic character. In fact last member of the group, bismuth hardly forms any compound in –3 oxidation state. The stability of +5 oxidation state decreases down the group. The only well characterised Bi (V) compound is BiF5. The stability of +5 oxidation state decreases and ...
... to increase in size and metallic character. In fact last member of the group, bismuth hardly forms any compound in –3 oxidation state. The stability of +5 oxidation state decreases down the group. The only well characterised Bi (V) compound is BiF5. The stability of +5 oxidation state decreases and ...
Mission Statement
... regular, repeatable patterns occur across periods and within groups on the periodic table Appreciate that these patterns sometimes have notable exceptions Recall and understand that the noble gases have full outer shells that represent stable electronic configurations Recall how, and understand elec ...
... regular, repeatable patterns occur across periods and within groups on the periodic table Appreciate that these patterns sometimes have notable exceptions Recall and understand that the noble gases have full outer shells that represent stable electronic configurations Recall how, and understand elec ...
Electron-scattering cross sections for 1
... To obtain more information on the electron scattering from the 1-pentene molecule, we have extended our experimental TCS studies towards the theoretical considerations concerning the elastic and ionization processes. We have calculated the ECS for the intermediate-energy (20–3000 eV) electron collis ...
... To obtain more information on the electron scattering from the 1-pentene molecule, we have extended our experimental TCS studies towards the theoretical considerations concerning the elastic and ionization processes. We have calculated the ECS for the intermediate-energy (20–3000 eV) electron collis ...
XI CHEMISTRY SAMPLE PAPER 4 Time: Three Hours
... Ans 7: Organic compound is fused with sodium metal to convert N, S, P and halogens present in organic compound to their corresponding sodium salts. (1 mark) ...
... Ans 7: Organic compound is fused with sodium metal to convert N, S, P and halogens present in organic compound to their corresponding sodium salts. (1 mark) ...
CO 2 - TrimbleChemistry
... Examples: Writing the Formula for Ionic Compounds • Ca and Cl • Ba and F • Na and S ...
... Examples: Writing the Formula for Ionic Compounds • Ca and Cl • Ba and F • Na and S ...
å¾è湿çå¦
... • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer. 7. Take care that you make firm, black pencil marks, just filling the oval. Be careful that any erasures are complete—make the ...
... • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer. 7. Take care that you make firm, black pencil marks, just filling the oval. Be careful that any erasures are complete—make the ...
CHAPTER-8 NCERT SOLUTIONS
... In sulphur dioxide (SO2), the oxidation number (O.N.) of S is +4 and the range of the O.N. that S can have is from +6 to –2. Therefore, SO2 can act as an oxidising as well as a reducing agent. In hydrogen peroxide (H2O2), the O.N. of O is –1 and the range of the O.N. that O can have is from 0 to –2. ...
... In sulphur dioxide (SO2), the oxidation number (O.N.) of S is +4 and the range of the O.N. that S can have is from +6 to –2. Therefore, SO2 can act as an oxidising as well as a reducing agent. In hydrogen peroxide (H2O2), the O.N. of O is –1 and the range of the O.N. that O can have is from 0 to –2. ...
Unit - 7.pmd
... to increase in size and metallic character. In fact last member of the group, bismuth hardly forms any compound in –3 oxidation state. The stability of +5 oxidation state decreases down the group. The only well characterised Bi (V) compound is BiF5. The stability of +5 oxidation state decreases and ...
... to increase in size and metallic character. In fact last member of the group, bismuth hardly forms any compound in –3 oxidation state. The stability of +5 oxidation state decreases down the group. The only well characterised Bi (V) compound is BiF5. The stability of +5 oxidation state decreases and ...
CHEMISTRY
... maintain the correct pH compounds containing hydrogencarbonate ions, HCO3-(aq), can be added. i. Write the equations which show how hydrogencarbonate ions can control the pH within the region given. ...
... maintain the correct pH compounds containing hydrogencarbonate ions, HCO3-(aq), can be added. i. Write the equations which show how hydrogencarbonate ions can control the pH within the region given. ...
Subject Area Standard Area Organizing Category Grade Level
... CHEM.A.2.2.2: Predict characteristics of an atom or an ion based on its location on the periodic table (e.g., number of valence electrons, potential types of bonds, reactivity). ...
... CHEM.A.2.2.2: Predict characteristics of an atom or an ion based on its location on the periodic table (e.g., number of valence electrons, potential types of bonds, reactivity). ...
Main-group elements as transition metals
... two non-bonded electron pairs. Again, the amount of geometrical distortion (that is, bending) increases as the group is descended, and it is possible to write these distorted structures using a valencebond approach (Fig. 1d), analogous to Lappert’s representations of the ethylene analogues (Fig. 1b ...
... two non-bonded electron pairs. Again, the amount of geometrical distortion (that is, bending) increases as the group is descended, and it is possible to write these distorted structures using a valencebond approach (Fig. 1d), analogous to Lappert’s representations of the ethylene analogues (Fig. 1b ...
1ST CHAPTER Long-questions-basic-concept
... existence is called an atom. For example ,the atoms of He,Ne and A r exist independently while the atoms of hydrogen ,nitrogen and oxygen do not have independent existence .An atom is composed of more than 100 subatomic particles such as electron, proton , neutron , hyperons , neutrino, antineutrino ...
... existence is called an atom. For example ,the atoms of He,Ne and A r exist independently while the atoms of hydrogen ,nitrogen and oxygen do not have independent existence .An atom is composed of more than 100 subatomic particles such as electron, proton , neutron , hyperons , neutrino, antineutrino ...
AP Chemistry Notes and Worksheets 2014
... covalent bonds-created when two or more nonmetals share electrons molecule- atoms held together by covalent bonds ions- charged particles formed by the loss or gain of electrons ionic bonds- compounds created when one atom loses an electron and another gains it; are held together by electros ...
... covalent bonds-created when two or more nonmetals share electrons molecule- atoms held together by covalent bonds ions- charged particles formed by the loss or gain of electrons ionic bonds- compounds created when one atom loses an electron and another gains it; are held together by electros ...
Chemistry Skills Practice Assignments
... 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allows you to tell? 4. Assuming that the temperature scales for both phase diagrams are the same, which c ...
... 2. For which substance, A or B, does the freezing point decrease as the pressure is increased? 3. One of the substances behaves more like most other substances. Which substance and what property allows you to tell? 4. Assuming that the temperature scales for both phase diagrams are the same, which c ...