Problem set 7
... ψ = u + v and ψ = u + iv in (1) and add the two resulting equations. Show that this reduces to Auv = (Avu )∗ . Thus the reality of expectation values in all states implies that A is hermitian in the conventional sense. The converse is much simpler. 5. Consider a particle in a (real) potential V(x). ...
... ψ = u + v and ψ = u + iv in (1) and add the two resulting equations. Show that this reduces to Auv = (Avu )∗ . Thus the reality of expectation values in all states implies that A is hermitian in the conventional sense. The converse is much simpler. 5. Consider a particle in a (real) potential V(x). ...
QM_2_particles_ver2
... unpaired electrons). 2. For a given multiplicity, the term with the largest value of L (orbital angular momentum), has the lowest energy 3. The level with lowest energy (where J=L+S) 1. Outer shell Less than half filled: minimum J 2. Outer shell more than half filled: maximum J ...
... unpaired electrons). 2. For a given multiplicity, the term with the largest value of L (orbital angular momentum), has the lowest energy 3. The level with lowest energy (where J=L+S) 1. Outer shell Less than half filled: minimum J 2. Outer shell more than half filled: maximum J ...
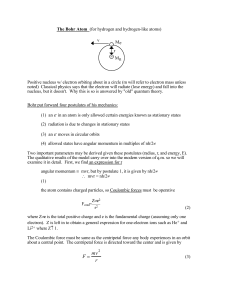
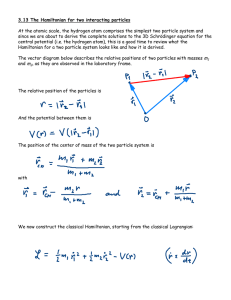
3.13 The Hamiltonian for two interacting particles At the atomic scale
... At the atomic scale, the hydrogen atom comprises the simplest two particle system and since we are about to derive the complete solutions to the 3D Schrödinger equation for the central potential (i.e. the hydrogen atom), this is a good time to review what the Hamiltonian for a two particle system lo ...
... At the atomic scale, the hydrogen atom comprises the simplest two particle system and since we are about to derive the complete solutions to the 3D Schrödinger equation for the central potential (i.e. the hydrogen atom), this is a good time to review what the Hamiltonian for a two particle system lo ...
Lecture 8 1 Schrodinger equation (continued)
... for an electron in an atom. To know this then we must make some assumptions about how electrons behave in an atom. Let’s assume that atoms are very tiny (≈ 10−10 meter) 1-D boxes with very hard walls. The walls are located at position x = 0 and x = l. This model works surprisingly well. Inside the b ...
... for an electron in an atom. To know this then we must make some assumptions about how electrons behave in an atom. Let’s assume that atoms are very tiny (≈ 10−10 meter) 1-D boxes with very hard walls. The walls are located at position x = 0 and x = l. This model works surprisingly well. Inside the b ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... U U 0 n 0 (0) n1 (1 0 ) n 2 ( 2 0 ) ..... - The equation of partition function for the particle at i=0 ...
... U U 0 n 0 (0) n1 (1 0 ) n 2 ( 2 0 ) ..... - The equation of partition function for the particle at i=0 ...
Quantum Mechanics
... Electron density goes away from the internuclear region! Destructive interference! ...
... Electron density goes away from the internuclear region! Destructive interference! ...
Electron configuration Jeopardy
... 400 – Why don’t you see a baseball travel in waves? Wave nature is inversely related to mass. So if something is big, you don’t see the wave characteristic. 500 – What is the difference between a continuous spectrum and a line spectrum and give an example of where you could find each. Continuous spe ...
... 400 – Why don’t you see a baseball travel in waves? Wave nature is inversely related to mass. So if something is big, you don’t see the wave characteristic. 500 – What is the difference between a continuous spectrum and a line spectrum and give an example of where you could find each. Continuous spe ...
Announcements
... square of what is called the wave function ψ(x) l The wave function is a solution to an equation called the Schroedinger equation ...
... square of what is called the wave function ψ(x) l The wave function is a solution to an equation called the Schroedinger equation ...
PHYS 481/681 Quantum Mechanics Stephen Lepp August 29, 2016
... Introduction to Quantum Mechanics nd the interpretation of its solutions, the uncertainty principles, one-dimensional problems, harmonic oscillator, angular momentum, the hydrogen atom. 3 credits. • Class MW 11:30-12:45 BPB 249. • Office Hours TTh 12:45-1:30 or by arrangement. • Textbook “Quantum Me ...
... Introduction to Quantum Mechanics nd the interpretation of its solutions, the uncertainty principles, one-dimensional problems, harmonic oscillator, angular momentum, the hydrogen atom. 3 credits. • Class MW 11:30-12:45 BPB 249. • Office Hours TTh 12:45-1:30 or by arrangement. • Textbook “Quantum Me ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.