1 Reduced Mass Coordinates
... Finite angular momentum. When l > 0, we have to require that A1 = 0 (because of Eq. 47) which at first appears to force all coefficients to be zero. However, when we reach p = l, where l = 1, 2, 3, ..., the coefficient multiplying Ap+1 (or Al+1 ) is zero and the recursion is satisfied for non-zero A ...
... Finite angular momentum. When l > 0, we have to require that A1 = 0 (because of Eq. 47) which at first appears to force all coefficients to be zero. However, when we reach p = l, where l = 1, 2, 3, ..., the coefficient multiplying Ap+1 (or Al+1 ) is zero and the recursion is satisfied for non-zero A ...
Chapter 27 Powerpoint
... would be emitted when a quantized oscillator jumped from one energy level to the next lower one ...
... would be emitted when a quantized oscillator jumped from one energy level to the next lower one ...
... Given two systems of N1 ≈ N2 = 1022 spins with multiplicity functions g1(N1,s1) and g2(N2,s-s1), the product g1g2 as a function of s1 is relatively sharply peaked at s1 = ŝ1. For s1 = ŝ1 + 1012, the product g1g2 is reduced by 10-174 from its peak value. Use the Gaussian approximation of the multipli ...
Final Review
... wavefunction is the Spherical Harmonic which is governed by 2 quantum #s. What are the allowed values for those two quantum #s. What are the forms of the eigenvalues for the energy of a rigid rotator? Be able to calculate the energy of any level or the difference in the energy between two levels. Wh ...
... wavefunction is the Spherical Harmonic which is governed by 2 quantum #s. What are the allowed values for those two quantum #s. What are the forms of the eigenvalues for the energy of a rigid rotator? Be able to calculate the energy of any level or the difference in the energy between two levels. Wh ...
Chapter 4-2 The Quantum Model of the Atom
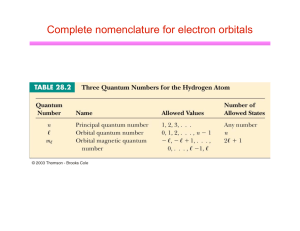
... Angular Momentum Quantum Number Except at the first main energy level, orbitals of different shapes exist for a given value of n. The angular momentum quantum number, symbolized by l, indicates the shape of the orbital. The number of orbital shapes possible is equal to n. The values of l allo ...
... Angular Momentum Quantum Number Except at the first main energy level, orbitals of different shapes exist for a given value of n. The angular momentum quantum number, symbolized by l, indicates the shape of the orbital. The number of orbital shapes possible is equal to n. The values of l allo ...
Elements of Dirac Notation
... electron densities, how the electrons are distributed in space in atoms and molecules. However, quantum mechanics has an equivalent formulation in momentum space. It answers the question of what does the distribution of electron velocities look like? The two formulations are equivalent, that is, the ...
... electron densities, how the electrons are distributed in space in atoms and molecules. However, quantum mechanics has an equivalent formulation in momentum space. It answers the question of what does the distribution of electron velocities look like? The two formulations are equivalent, that is, the ...
Wednesday, March 3, 2010
... The potentials and the Schrödinger wave equation for the three regions are as follows: ...
... The potentials and the Schrödinger wave equation for the three regions are as follows: ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.