Conservation of Energy
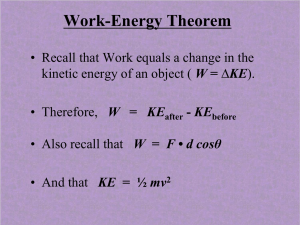
... • We stated earlier that when one object does work on another it changes the motion (kinetic energy) of the second object. • This relationship between work and kinetic energy is known as the Work-Kinetic Energy Theorem and can be expressed as: ...
... • We stated earlier that when one object does work on another it changes the motion (kinetic energy) of the second object. • This relationship between work and kinetic energy is known as the Work-Kinetic Energy Theorem and can be expressed as: ...
Conservation of Mechanical Energy Law of Conservation of Energy
... Astronomers discover a meteorite at a distance of 80,000 km from the center of Earth. The meteorite is moving directly toward Earth with a velocity of 2000 m/s What will be the velocity of the meteorite when it hits Earth’s surface? Ignore all drag effects. ...
... Astronomers discover a meteorite at a distance of 80,000 km from the center of Earth. The meteorite is moving directly toward Earth with a velocity of 2000 m/s What will be the velocity of the meteorite when it hits Earth’s surface? Ignore all drag effects. ...
The history of thoughta and science
... complexity of the world, to examine it piece by piece, and to build it up with deeper understanding. On the other hand, Greeks, especially Aristotle, heavily relied on speculation about the nature instead of experimental observation. They also lacked the quantitative explanatory power in their theor ...
... complexity of the world, to examine it piece by piece, and to build it up with deeper understanding. On the other hand, Greeks, especially Aristotle, heavily relied on speculation about the nature instead of experimental observation. They also lacked the quantitative explanatory power in their theor ...
Conservation of Mechanical Energy Law of Conservation of Energy
... ∴ ΔK + ΔU + Δ[all other forms of energy ]= 0 • Includes thermodynamic energy, strain energy, chemical energy, etc. ...
... ∴ ΔK + ΔU + Δ[all other forms of energy ]= 0 • Includes thermodynamic energy, strain energy, chemical energy, etc. ...
Historic Development by Mihai
... Einstein came up with the hypothesis of the corpuscular nature of light explaining the photoelectric effect. Einstein theory was verified by Millikan experiments (1916) ,he received Nobel price for that. Careful investigations toward the end of the nineteenth century proved that the photoelectric ef ...
... Einstein came up with the hypothesis of the corpuscular nature of light explaining the photoelectric effect. Einstein theory was verified by Millikan experiments (1916) ,he received Nobel price for that. Careful investigations toward the end of the nineteenth century proved that the photoelectric ef ...
THINGSYOUNEEDTOKNOW-modern
... ELECTRONS can collide with PHOTONS. Their combined momentums and energies are conserved. If Electron losses energy, thus losses speed If photon losses energy, its frequency decreases Because photon is light and must travel at c. The sum of all mass + energy is conserved in the universe. The sum of a ...
... ELECTRONS can collide with PHOTONS. Their combined momentums and energies are conserved. If Electron losses energy, thus losses speed If photon losses energy, its frequency decreases Because photon is light and must travel at c. The sum of all mass + energy is conserved in the universe. The sum of a ...
Please look over the following review questions
... An electron and a baseball move at the same speed. Which has the longer ...
... An electron and a baseball move at the same speed. Which has the longer ...
Standards SP1. Students will analyze the relationships between
... quantum mechanics and relativity when matter is very small, moving fast compared to the speed of light, or very large. b. Describe the Uncertainty Principle. c. Explain the differences in time, space, ...
... quantum mechanics and relativity when matter is very small, moving fast compared to the speed of light, or very large. b. Describe the Uncertainty Principle. c. Explain the differences in time, space, ...
Standard EPS Shell Presentation
... paint, and everything else around you come from this property of elements to emit or absorb light of only certain colors. ...
... paint, and everything else around you come from this property of elements to emit or absorb light of only certain colors. ...
Unit 4-3 Noteguide Phsyics and Quantem Mechanical
... Similar to how two snowflakes are not the same --emission spectrum useful to study the stars’ make-up Bohr’s model also hypothesized that electrons can jump to higher energy levels. (hydrogen atom only) --electron in its lowest energy level = ground state (quantum # (n) = 1) --when the electron gets ...
... Similar to how two snowflakes are not the same --emission spectrum useful to study the stars’ make-up Bohr’s model also hypothesized that electrons can jump to higher energy levels. (hydrogen atom only) --electron in its lowest energy level = ground state (quantum # (n) = 1) --when the electron gets ...
CHAPTER 1. SECOND QUANTIZATION In Chapter 1, F&W explain the basic theory: ❖
... Let Ψ(x,t) be the field operator for a spin-½ fermion, in the Heisenberg picture. Derive the field equation for Ψ(x,t), in the form iħ ∂Ψ / ∂t = F[Ψ] , where F[ Ψ] is a functional― which may involve derivatives and integrals. Simplify the result as much as possible. [[Assume that T(x) = - ħ2∇2 /2m a ...
... Let Ψ(x,t) be the field operator for a spin-½ fermion, in the Heisenberg picture. Derive the field equation for Ψ(x,t), in the form iħ ∂Ψ / ∂t = F[Ψ] , where F[ Ψ] is a functional― which may involve derivatives and integrals. Simplify the result as much as possible. [[Assume that T(x) = - ħ2∇2 /2m a ...
Introduction to Statistical Thermodynamics - cryocourse 2011
... Fundamental Postulate: An isolated system in equilibrium has the same probability to be found in any of its accessible states. - One can show that this probability does not evolve with time. - This leads to results that agree remarkably well with experiments. - Equilibrium is achieved through “smal ...
... Fundamental Postulate: An isolated system in equilibrium has the same probability to be found in any of its accessible states. - One can show that this probability does not evolve with time. - This leads to results that agree remarkably well with experiments. - Equilibrium is achieved through “smal ...
Study Guide - Rose
... 1. What is the Schroedinger equation and what is it used for? 2. What is the normalization condition and why is it important? 3. Can a wavefunction be measured directly for a particle? If not, what can be measured directly? 4. List and describe the 4 conditions that a wavefunction must satisfy in or ...
... 1. What is the Schroedinger equation and what is it used for? 2. What is the normalization condition and why is it important? 3. Can a wavefunction be measured directly for a particle? If not, what can be measured directly? 4. List and describe the 4 conditions that a wavefunction must satisfy in or ...