Lecture 19
... work can be converted into heat! Therefore, heat is one of the forms of energy. It led to the First Law of Thermodynamics It also showed that energy is conserved. ...
... work can be converted into heat! Therefore, heat is one of the forms of energy. It led to the First Law of Thermodynamics It also showed that energy is conserved. ...
Slide 1
... historic paper ends with the statements: I will therefore conclude by considering it as demonstrated by the experiments contained in this paper: 1.That the quantity of heat produced by the friction of bodies, whether solid or liquid, is always proportional to the quantity of force extended. 2.That t ...
... historic paper ends with the statements: I will therefore conclude by considering it as demonstrated by the experiments contained in this paper: 1.That the quantity of heat produced by the friction of bodies, whether solid or liquid, is always proportional to the quantity of force extended. 2.That t ...
week_9_homework_work_and_energy_conservation_markscheme
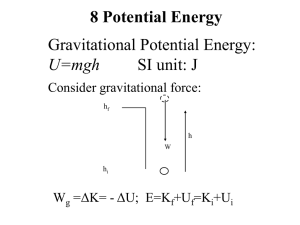
... How speed at C would be expected to differ from previous answer: Same speed/no effect [If this is wrong, no marks] (1) GPE and KE both symbol 181 \f “ 12µ m OR g same for all masses OR ms cancel (1) ...
... How speed at C would be expected to differ from previous answer: Same speed/no effect [If this is wrong, no marks] (1) GPE and KE both symbol 181 \f “ 12µ m OR g same for all masses OR ms cancel (1) ...
sgt2
... B. You should be able to define the following: weight, work, power, kinetic energy, potential energy, mechanical energy, conservative, and non-conservative forces. C. The following equations will be provided if needed: 1. Equations of kinematics ...
... B. You should be able to define the following: weight, work, power, kinetic energy, potential energy, mechanical energy, conservative, and non-conservative forces. C. The following equations will be provided if needed: 1. Equations of kinematics ...
Review of Work and Energy
... Fg = mg The work done by non-conservative (or external) forces can be calculated in the way one calculates the work done by any individual force, as shown above, or using the work – energy equation: ...
... Fg = mg The work done by non-conservative (or external) forces can be calculated in the way one calculates the work done by any individual force, as shown above, or using the work – energy equation: ...
3.012 Practice Problems for Recitation 1 (09.13.05) Part I. System
... Imagine a process for each (real or idealized). Compare (qualitatively) the rates of change for the thermodynamic variables in the two processes. The two imagined processes would start from the same basic components: a cylinder of gas and a piston. For the reversible process, the piston would move v ...
... Imagine a process for each (real or idealized). Compare (qualitatively) the rates of change for the thermodynamic variables in the two processes. The two imagined processes would start from the same basic components: a cylinder of gas and a piston. For the reversible process, the piston would move v ...
Verdana 30 pt - Liceo Statale Aprosio
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...