first chapter - damtp - University of Cambridge
... An atom consists of a positively charged nucleus, together with a number of negatively charged electrons. Inside the nucleus there are protons, each of which carries positive charge e, and neutrons, which have no charge. So the charge on the nucleus is Ze, where Z , the atomic number, is the number ...
... An atom consists of a positively charged nucleus, together with a number of negatively charged electrons. Inside the nucleus there are protons, each of which carries positive charge e, and neutrons, which have no charge. So the charge on the nucleus is Ze, where Z , the atomic number, is the number ...
schrodinger
... •Divide by the t 2m x original wave function •Note that left side is independent of x, and right side is independent of t. •Both sides must be independent of both x and t •Both sides must be equal to a constant, called E (the energy) ...
... •Divide by the t 2m x original wave function •Note that left side is independent of x, and right side is independent of t. •Both sides must be independent of both x and t •Both sides must be equal to a constant, called E (the energy) ...
CHAPTER 7: The Hydrogen Atom
... The Dutch physicist Pieter Zeeman showed the spectral lines emitted by atoms in a magnetic field split into multiple energy levels. It is called the Zeeman effect. A spectral line is split into three lines. Consider the atom to behave like a small magnet. Think of an electron as an orbiting circular ...
... The Dutch physicist Pieter Zeeman showed the spectral lines emitted by atoms in a magnetic field split into multiple energy levels. It is called the Zeeman effect. A spectral line is split into three lines. Consider the atom to behave like a small magnet. Think of an electron as an orbiting circular ...
Downlad - Inspiron Technologies
... The Heisenberg uncertainty principle states: if a measurement of the position of a particle is made with uncertainty Dx and a simultaneous measurement of its x component of momentum is made with uncertainty Dpx, the product of the two uncertainties can never be smaller than /2 ...
... The Heisenberg uncertainty principle states: if a measurement of the position of a particle is made with uncertainty Dx and a simultaneous measurement of its x component of momentum is made with uncertainty Dpx, the product of the two uncertainties can never be smaller than /2 ...
Document
... of conservative forces that do not depend on time t explicitly. In this case there exists the energy integral H = T U = ε where U ( q1 , ..., qn ) is potential energy, q1 ,...,qk are coordinates, p1 ,..., pk are momenta, and 2T = aij pi p j the kinetic energy of the system. i, j The complete integ ...
... of conservative forces that do not depend on time t explicitly. In this case there exists the energy integral H = T U = ε where U ( q1 , ..., qn ) is potential energy, q1 ,...,qk are coordinates, p1 ,..., pk are momenta, and 2T = aij pi p j the kinetic energy of the system. i, j The complete integ ...
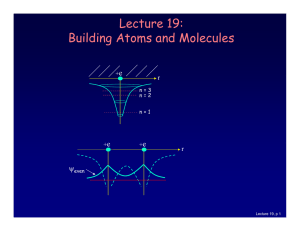
stationary state
... • When an electron is in one of the quantized orbits, it does not emit any electromagnetic radiation; thus, the electron is said to be in a stationary state. • The electron can make a discontinuous emission, or quantum jump, from one stationary state to another. During this transition it does emit r ...
... • When an electron is in one of the quantized orbits, it does not emit any electromagnetic radiation; thus, the electron is said to be in a stationary state. • The electron can make a discontinuous emission, or quantum jump, from one stationary state to another. During this transition it does emit r ...
The Klein-Gordon equation
... Charge and charge conjugation Equations (80) - (83) show that the Q-eigenvalues have the same properties as the electric charge measured in units of e : they are integers, additive and zero for the vacuum. The complex free Klein-Gordon field therefore has a charge-like quantum number! In the case o ...
... Charge and charge conjugation Equations (80) - (83) show that the Q-eigenvalues have the same properties as the electric charge measured in units of e : they are integers, additive and zero for the vacuum. The complex free Klein-Gordon field therefore has a charge-like quantum number! In the case o ...
PL-sp06-m14-Mesoscale
... – Pretty fast due to small size, but probably... • ~1000’s × slower than molecular electronics might be – basically, because atoms are ~1000’s × heavier than electrons ...
... – Pretty fast due to small size, but probably... • ~1000’s × slower than molecular electronics might be – basically, because atoms are ~1000’s × heavier than electrons ...
Hwk Set #14 - Publisher`s solutions
... know the emitted photon has energy Ephoton = hf photon = ΔE. Combine these to get ωe = 2π λc . Solve: ...
... know the emitted photon has energy Ephoton = hf photon = ΔE. Combine these to get ωe = 2π λc . Solve: ...