Document
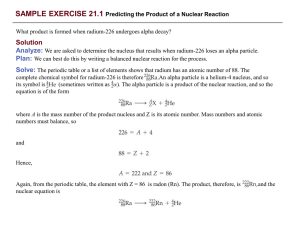
... Plan: We can best do this by writing a balanced nuclear reaction for the process. Solve: The periodic table or a list of elements shows that radium has an atomic number of 88. The complete chemical symbol for radium-226 is therefore An alpha particle is a helium-4 nucleus, and so ...
... Plan: We can best do this by writing a balanced nuclear reaction for the process. Solve: The periodic table or a list of elements shows that radium has an atomic number of 88. The complete chemical symbol for radium-226 is therefore An alpha particle is a helium-4 nucleus, and so ...
thermodynamic - Portal UniMAP
... random movement of the molecules) Temperature (average amount of kinetic energy result from random movement of the molecules) ...
... random movement of the molecules) Temperature (average amount of kinetic energy result from random movement of the molecules) ...
The concepts of an atom and chemical bond in physics and chemistry
... species in many practical cases. What is more – such „chemical” picture of atoms and bonds prevails in chemical education and scientific research although a quite different view of these concepts emerges from extremely successful application of quantum mechanical formalism to many chemical problems. ...
... species in many practical cases. What is more – such „chemical” picture of atoms and bonds prevails in chemical education and scientific research although a quite different view of these concepts emerges from extremely successful application of quantum mechanical formalism to many chemical problems. ...
Document
... K, each line characterized by a certain J value must have 2J + 1 components. However, since K only appears as K2 in the equation, there will be only J + 1 different frequencies all those with K > 0 being doubly degenerate. Note: each spectrum yields only one value of B, but the spectra of isotopic ...
... K, each line characterized by a certain J value must have 2J + 1 components. However, since K only appears as K2 in the equation, there will be only J + 1 different frequencies all those with K > 0 being doubly degenerate. Note: each spectrum yields only one value of B, but the spectra of isotopic ...
Photons and Matter Waves
... certain metal with a maximum kinetic energy of 2 eV. If photons of twice the wavelength are incident on this metal which one of the following statements is true? 1) No electrons will be emitted. 2) Electrons will be emitted with a maximum kinetic energy of 1 eV. 3) Electrons will be emitted with a m ...
... certain metal with a maximum kinetic energy of 2 eV. If photons of twice the wavelength are incident on this metal which one of the following statements is true? 1) No electrons will be emitted. 2) Electrons will be emitted with a maximum kinetic energy of 1 eV. 3) Electrons will be emitted with a m ...
Quantum Chemistry - Winona State University
... Can only explain the line spectrum of hydrogen adequately. Can only work for (at least) one electron atoms. Cannot explain multi-lines with each color. Cannot explain relative intensities. ...
... Can only explain the line spectrum of hydrogen adequately. Can only work for (at least) one electron atoms. Cannot explain multi-lines with each color. Cannot explain relative intensities. ...
CHEM 121 Chp 2 Spaulding
... latching onto many other atoms (including itself) and holding tight, forming molecular conga lines of hearty robustness-the very trick of nature necessary to build proteins and DNA” ◦ Bill Bryson from A Short History of Nearly Everything ...
... latching onto many other atoms (including itself) and holding tight, forming molecular conga lines of hearty robustness-the very trick of nature necessary to build proteins and DNA” ◦ Bill Bryson from A Short History of Nearly Everything ...
Electronic and atomic structure of liquid potassium via
... In order to produce an effective classical potential useful in a computer simulation, one would like to rewrite (or approximate) the partition function in a way that corresponds to a sum of positive terms. Such an approximation may be possible for some systems provided the terms with positive weight ...
... In order to produce an effective classical potential useful in a computer simulation, one would like to rewrite (or approximate) the partition function in a way that corresponds to a sum of positive terms. Such an approximation may be possible for some systems provided the terms with positive weight ...
Chemistry MSL Practical Style Review 1. What is the nuclear
... increasing the temperature increases the rate of the reaction. Which is the best explanation for this happening? A B C D ...
... increasing the temperature increases the rate of the reaction. Which is the best explanation for this happening? A B C D ...
CH 28 – Atomic Physics
... protons and neutrons with electrons in orbits about the nucleus. The neutron has no charge and the number of protons and electrons are the same so that the atom has no net charge. The diameter of the nucleus is much smaller than the diameter of the atom, but the nucleus contains most of the mass of ...
... protons and neutrons with electrons in orbits about the nucleus. The neutron has no charge and the number of protons and electrons are the same so that the atom has no net charge. The diameter of the nucleus is much smaller than the diameter of the atom, but the nucleus contains most of the mass of ...
Atomic Physics
... protons and neutrons with electrons in orbits about the nucleus. The neutron has no charge and the number of protons and electrons are the same so that the atom has no net charge. The diameter of the nucleus is much smaller than the diameter of the atom, but the nucleus contains most of the mass of ...
... protons and neutrons with electrons in orbits about the nucleus. The neutron has no charge and the number of protons and electrons are the same so that the atom has no net charge. The diameter of the nucleus is much smaller than the diameter of the atom, but the nucleus contains most of the mass of ...
PDF
... In the quantum algorithm, what we want to do is to use the fact that there are an equal number of 0s and 1s, to get the 0s and 1s to cancel one another. First, however, we need to be clear as to what exactly is given in the quantum algorithm. The quantum algorithm does not oracle-query f , rather it ...
... In the quantum algorithm, what we want to do is to use the fact that there are an equal number of 0s and 1s, to get the 0s and 1s to cancel one another. First, however, we need to be clear as to what exactly is given in the quantum algorithm. The quantum algorithm does not oracle-query f , rather it ...
Measuring and Calculating
... substance that increases the rate of a reaction by lowering the activation energy and is not consumed by the reaction ...
... substance that increases the rate of a reaction by lowering the activation energy and is not consumed by the reaction ...
Coulomb Drag to Measure Electron-Electron Interaction in Bilayer
... Notice that individual layer scattering times are going to disappear from the ratio between E1 and I2. This is immensely important - because we have now related a transport measurement to electron-electron scattering . The effect of disorder has somehow disappeared - at least within the relaxation t ...
... Notice that individual layer scattering times are going to disappear from the ratio between E1 and I2. This is immensely important - because we have now related a transport measurement to electron-electron scattering . The effect of disorder has somehow disappeared - at least within the relaxation t ...