Introduction to P880.P20
... b) To what precision do we need to measure the momentum of the p and k? will need a magnet will need to measure trajectory in magnetic field c) Do we need to use a calorimeter to measure energy? d) How will we know that only an X+ is produced? e) How much space do we have for the experiment? f) How ...
... b) To what precision do we need to measure the momentum of the p and k? will need a magnet will need to measure trajectory in magnetic field c) Do we need to use a calorimeter to measure energy? d) How will we know that only an X+ is produced? e) How much space do we have for the experiment? f) How ...
PDF sample
... gathered together such that they think that they are you. Particle physics is the subject that has shown how matter is built and which is beginning to explain where it all came from. In huge accelerators, often several miles in length, we can speed pieces of atoms, particles such as electrons and pr ...
... gathered together such that they think that they are you. Particle physics is the subject that has shown how matter is built and which is beginning to explain where it all came from. In huge accelerators, often several miles in length, we can speed pieces of atoms, particles such as electrons and pr ...
Passive Dynamics and Particle Systems COS 426, Spring 2014 Princeton University
... • Collision detection Intersect ray with scene Compute up to Δt at time of first collision, and then continue from there ...
... • Collision detection Intersect ray with scene Compute up to Δt at time of first collision, and then continue from there ...
Document
... Quantum field theory describes the quantum mechanics of fields, such as the electromagnetic field and the electron field. In this setup, particles and waves, both are different faces of the same type of object: the quantum field. Feynman’s pictorial representation of possibilities in the bubble of i ...
... Quantum field theory describes the quantum mechanics of fields, such as the electromagnetic field and the electron field. In this setup, particles and waves, both are different faces of the same type of object: the quantum field. Feynman’s pictorial representation of possibilities in the bubble of i ...
nuclear physics in the vedas
... atoms combine with each other to produce everything in the universe only because of this interaction “eating” – and the “leftovers” (उिSछ ठात जW9रे सवम – अथववेद - 11-9-11). We enjoy the objects that are its “left over” - &वRय. For this reason, the Isha Upanishad says: ईशावा1यMमदम सवम. ...
... atoms combine with each other to produce everything in the universe only because of this interaction “eating” – and the “leftovers” (उिSछ ठात जW9रे सवम – अथववेद - 11-9-11). We enjoy the objects that are its “left over” - &वRय. For this reason, the Isha Upanishad says: ईशावा1यMमदम सवम. ...
Chemistry Vocabulary List
... charge, nearly all of the mass, but a very small fraction of the volume of an atom. 2. Proton => Positively charged particle located in the nucleus of an atom; charge = +1, mass = 1 atomic mass unit 3. Neutron => Neutrally charged particle located in the nucleus of an atom; charge = 0, mass = 1 atom ...
... charge, nearly all of the mass, but a very small fraction of the volume of an atom. 2. Proton => Positively charged particle located in the nucleus of an atom; charge = +1, mass = 1 atomic mass unit 3. Neutron => Neutrally charged particle located in the nucleus of an atom; charge = 0, mass = 1 atom ...
Charges Near Magnets—Magnetic Force184 The figures below
... The figures below show electrically charged particles sitting at rest near the poles of permanent magnets. All of the permanent magnets are the same strength. The magnitudes and signs of the electric charges are shown in the circles, which represent the particles. The zero values indicate that the p ...
... The figures below show electrically charged particles sitting at rest near the poles of permanent magnets. All of the permanent magnets are the same strength. The magnitudes and signs of the electric charges are shown in the circles, which represent the particles. The zero values indicate that the p ...
Heisenberg`s Uncertainty Principle
... We utterly reject the idea that particles can be created from nothing, exist for a short time and then fade from existence so long as δE δt ≤ h. Page 1 of 2 © Copyright Bruce Harvey 1997/2008 ...
... We utterly reject the idea that particles can be created from nothing, exist for a short time and then fade from existence so long as δE δt ≤ h. Page 1 of 2 © Copyright Bruce Harvey 1997/2008 ...
Chemical Building Blocks
... QUIZ: ½ sheet of paper An analyst measured a 3.3 x 10-2 L sample and found it weighed 385 mg. Report its density in g/mL. (Proper SF) Give an example of the ff. ...
... QUIZ: ½ sheet of paper An analyst measured a 3.3 x 10-2 L sample and found it weighed 385 mg. Report its density in g/mL. (Proper SF) Give an example of the ff. ...
QUIZ: History of Atomic Structure
... A) all nuclei contain protons B) all forms of matter contain electrons C) all positive rays were actually protons D) all alpha particles are heavier than protons 3. Many classic experiments have given us indirect evidence of the nature of the atom. Which of the experiments listed below did not give ...
... A) all nuclei contain protons B) all forms of matter contain electrons C) all positive rays were actually protons D) all alpha particles are heavier than protons 3. Many classic experiments have given us indirect evidence of the nature of the atom. Which of the experiments listed below did not give ...
Kscenario - Elementary Particle Physics Group
... quarks, like neutrons, also deposit energy in the hadronic calorimeter, but leave no track in the tracking ...
... quarks, like neutrons, also deposit energy in the hadronic calorimeter, but leave no track in the tracking ...
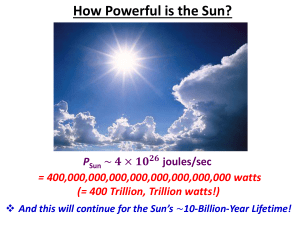
How Powerful is the Sun?
... And the tremendous penetrating power of Neutrinos helps us Study the Universe, today! ...
... And the tremendous penetrating power of Neutrinos helps us Study the Universe, today! ...
Project 1
... The Neutron is the neutral part of the atom Discovered by Walther Bothe, Irène Joliot-Curie, Frédéric Joliot, and James Chadwick in the early 1930’s The neutron has to do with the strong force which holds the atom together (gravity is too weak at this level to hold the nucleus together), but when an ...
... The Neutron is the neutral part of the atom Discovered by Walther Bothe, Irène Joliot-Curie, Frédéric Joliot, and James Chadwick in the early 1930’s The neutron has to do with the strong force which holds the atom together (gravity is too weak at this level to hold the nucleus together), but when an ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.