Fermium does not occur naturally in the Earth`s crust. It was first
... atomic number (Z) – The number of protons in the nucleus of an atom. decay product – (daughter product), any nuclide produced by a specified radionuclide (parent) in a decay chain. [return] electron – elementary particle of matter with a negative electric charge and a rest mass of about 9.109 × 10–3 ...
... atomic number (Z) – The number of protons in the nucleus of an atom. decay product – (daughter product), any nuclide produced by a specified radionuclide (parent) in a decay chain. [return] electron – elementary particle of matter with a negative electric charge and a rest mass of about 9.109 × 10–3 ...
here
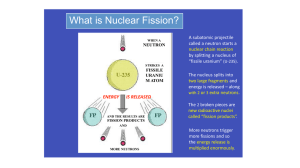
... with 2 or 3 extra neutrons. The 2 broken pieces are new radioactive nuclei called “fission products”. ...
... with 2 or 3 extra neutrons. The 2 broken pieces are new radioactive nuclei called “fission products”. ...
The Pauli-Exclusion Principle Indistinguishability
... between identical particles and spin. This principle, as we will see, is responsible for atomic structure (and thus all of chemistry!) and nuclear structure. However, the PauliExclusion Principle only applies to fermions. If the particles are bosons (integral spin) then they are not affected. You ca ...
... between identical particles and spin. This principle, as we will see, is responsible for atomic structure (and thus all of chemistry!) and nuclear structure. However, the PauliExclusion Principle only applies to fermions. If the particles are bosons (integral spin) then they are not affected. You ca ...
annotated_activity_list
... Steckbriefe (particle cards) - introduces concept of standard model, fundamental particles, and use of classifucation in science Rolling with Rutherford - introduces indirect measurement, statistics, histogram and its analysis, use of probability Quark Workbench - introduces quark combination rules, ...
... Steckbriefe (particle cards) - introduces concept of standard model, fundamental particles, and use of classifucation in science Rolling with Rutherford - introduces indirect measurement, statistics, histogram and its analysis, use of probability Quark Workbench - introduces quark combination rules, ...
Atoms
... The work of J.J. Thomson, E. Rutherford and others toward the end of the 19th century and into the beginning of the 20th century established that atoms consist of a positively charged nucleus and negatively charged electrons. In a neutral atom, the positive charge on the nucleus exactly balances the ...
... The work of J.J. Thomson, E. Rutherford and others toward the end of the 19th century and into the beginning of the 20th century established that atoms consist of a positively charged nucleus and negatively charged electrons. In a neutral atom, the positive charge on the nucleus exactly balances the ...
Diffusion of Individual Atoms
... In a scientific first, a team of researchers from Macquarie University and the University of Vienna have developed a new technique to measure molecular properties – forming the basis for improvements in scientific instruments like telescopes, and with the potential to speed up the development of pha ...
... In a scientific first, a team of researchers from Macquarie University and the University of Vienna have developed a new technique to measure molecular properties – forming the basis for improvements in scientific instruments like telescopes, and with the potential to speed up the development of pha ...
Open Questions in Physics
... quantum jumps, then I'm sorry that I ever got involved!” E.Schrodinger • Do there exist phenomena that are truly spontaneous? Or do all phenomena, when investigated in depth, turn out to be deterministic? ...
... quantum jumps, then I'm sorry that I ever got involved!” E.Schrodinger • Do there exist phenomena that are truly spontaneous? Or do all phenomena, when investigated in depth, turn out to be deterministic? ...
Inside A Particle Physicist`s Toolbox
... observed copious sparks Age 7 discovered the telegraph ...
... observed copious sparks Age 7 discovered the telegraph ...
Chemistry Student Guided Notes
... The periodic table of the elements is arranged so that a great deal of information about all of the known elements is provided in a small space. Generally, each element is identified by a one-, two-, or three-letter abbreviation known as a All elements are classified and arranged according to their ...
... The periodic table of the elements is arranged so that a great deal of information about all of the known elements is provided in a small space. Generally, each element is identified by a one-, two-, or three-letter abbreviation known as a All elements are classified and arranged according to their ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.