LOYOLA COLLEGE (AUTONOMOUS), CHENNAI M.Sc. SECOND
... 16. (a) A particle of mass m moves in a three dimensional box of sides a, b, c. If the potential is zero inside and infinity outside the box, find the energy eigen values and eigen functions. (b) If the box is a cubical one of side a, derive expression for energy eigen values and eigen functions. (9 ...
... 16. (a) A particle of mass m moves in a three dimensional box of sides a, b, c. If the potential is zero inside and infinity outside the box, find the energy eigen values and eigen functions. (b) If the box is a cubical one of side a, derive expression for energy eigen values and eigen functions. (9 ...
Formulae and Data Booklet - SCSA
... Copying or communication for any other purpose can be done only within the terms of the Copyright Act 1968 or with prior written permission of the School Curriculum and Standards Authority. Copying or communication of any third party copyright material can be done only within the terms of the Copyri ...
... Copying or communication for any other purpose can be done only within the terms of the Copyright Act 1968 or with prior written permission of the School Curriculum and Standards Authority. Copying or communication of any third party copyright material can be done only within the terms of the Copyri ...
Early Modern Physics
... Rutherford Scattering III • already went over kinematics • Rutherford scattering can either be off a heavier object (nuclei) change in angle but little energy loss “multiple scattering” • or off light target (electrons) where can transfer energy but little angular change (energy loss due to ion ...
... Rutherford Scattering III • already went over kinematics • Rutherford scattering can either be off a heavier object (nuclei) change in angle but little energy loss “multiple scattering” • or off light target (electrons) where can transfer energy but little angular change (energy loss due to ion ...
File
... Carried out in a Hoffman’s apparatus (shown to the right), it splits water compounds into oxygen molecules and hydrogen molecules Water Oxygen + Hydrogen H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matte ...
... Carried out in a Hoffman’s apparatus (shown to the right), it splits water compounds into oxygen molecules and hydrogen molecules Water Oxygen + Hydrogen H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matte ...
Serge Haroche
... The Nobel Laureates have opened the door to a new era of experimentation with quantum physics by demonstrating the direct observation of individual quantum particles without destroying them. For single particles of light or matter the laws of classical physics cease to apply and quantum physics take ...
... The Nobel Laureates have opened the door to a new era of experimentation with quantum physics by demonstrating the direct observation of individual quantum particles without destroying them. For single particles of light or matter the laws of classical physics cease to apply and quantum physics take ...
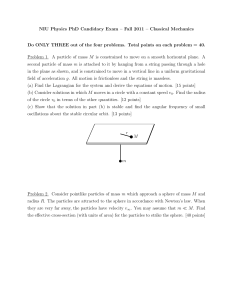
NIU Physics PhD Candidacy Exam – Fall 2011 – Classical
... radius R. The particles are attracted to the sphere in accordance with Newton’s law. When they are very far away, the particles have velocity v∞ . You may assume that m ¿ M . Find the effective cross-section (with units of area) for the particles to strike the sphere. [40 points] ...
... radius R. The particles are attracted to the sphere in accordance with Newton’s law. When they are very far away, the particles have velocity v∞ . You may assume that m ¿ M . Find the effective cross-section (with units of area) for the particles to strike the sphere. [40 points] ...
An Introduction to particle Accelerators
... H. Wiedemann, “Particle Accelerator Physics,” (2 volumes), Springer 1993 K. Wille, “The Physics of Particle Accelerators : An Introduction,” ...
... H. Wiedemann, “Particle Accelerator Physics,” (2 volumes), Springer 1993 K. Wille, “The Physics of Particle Accelerators : An Introduction,” ...
What does LHC stand for
... What are the main goals of the LHC? Our current understanding of the Universe is incomplete. The Standard Model of particles and forces l has been tested by various experiments and it has proven particularly successful in anticipating the existence of previously undiscovered particles. However, it l ...
... What are the main goals of the LHC? Our current understanding of the Universe is incomplete. The Standard Model of particles and forces l has been tested by various experiments and it has proven particularly successful in anticipating the existence of previously undiscovered particles. However, it l ...
Ch. 02 Powerpoint Overview Assignment Page
... * John Dalton’s proposal by 1808 to explain major Laws * Consists of several postulates: I: Atoms are the smallest, indivisible particles of matter still retaining a chemical identity. II: All atoms of an element are identical. ...
... * John Dalton’s proposal by 1808 to explain major Laws * Consists of several postulates: I: Atoms are the smallest, indivisible particles of matter still retaining a chemical identity. II: All atoms of an element are identical. ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... The sum of the number of neutrons and protons in the nucleus of an atom. Nucleus The small “core” of the atom, where most of its mass and all of its positive charge is concentrated. Except for ordinary hydrogen (which has only a proton), atomic nuclei consist of protons and neutrons. For this reason ...
... The sum of the number of neutrons and protons in the nucleus of an atom. Nucleus The small “core” of the atom, where most of its mass and all of its positive charge is concentrated. Except for ordinary hydrogen (which has only a proton), atomic nuclei consist of protons and neutrons. For this reason ...
Slide 1
... Quantum dots A quantum dot is in analogy to the “particle in a box” model, where ΔE increases with decreasing a. ...
... Quantum dots A quantum dot is in analogy to the “particle in a box” model, where ΔE increases with decreasing a. ...
Single Particles Do Not Exhibit Wave-like Behavior
... The Davisson-Germer experiment is perceived as that which proved the wave-like behavior of the particle in the relationship between the particle's momentum P and its de Broglie wave length ~ P=h/ . However, in view of the above analysis, a single particle will not exhibit wave-like behavior, but onl ...
... The Davisson-Germer experiment is perceived as that which proved the wave-like behavior of the particle in the relationship between the particle's momentum P and its de Broglie wave length ~ P=h/ . However, in view of the above analysis, a single particle will not exhibit wave-like behavior, but onl ...
ppt
... The vertical ripples make it look like 1 km thick. Because of the thinness, the optical depth is only 0.1-2. Optical depth = thickness / mean free path Mean free path = (n ) 1, where n = particle number density = cross section = a2 The rings are thin because inelastic collisions between partic ...
... The vertical ripples make it look like 1 km thick. Because of the thinness, the optical depth is only 0.1-2. Optical depth = thickness / mean free path Mean free path = (n ) 1, where n = particle number density = cross section = a2 The rings are thin because inelastic collisions between partic ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.