physical science 9.4
... State that infra-red radiation, a form of light and a part of the Electromagnetic Spectrum, reaches us from the Sun through Space as a waveform. Convection and conduction are not possible in Space. ...
... State that infra-red radiation, a form of light and a part of the Electromagnetic Spectrum, reaches us from the Sun through Space as a waveform. Convection and conduction are not possible in Space. ...
Inside the Atom connections to the lower secondary (KS3
... • a simple (Dalton) atomic model • differences between atoms, elements and compounds • chemical symbols and formulae for elements and compounds • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemi ...
... • a simple (Dalton) atomic model • differences between atoms, elements and compounds • chemical symbols and formulae for elements and compounds • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemi ...
Unit 8 Waves: Quantum Mechanical Waves
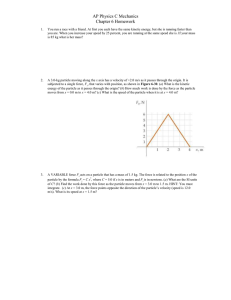
... The yellow-white light of the Sun (familiar to all) arises because the Sun is very hot and emits light at all visible wavelengths. However, in 1802, studies with prisms (similar to what was used in the laboratory) showed gaps in the Sun’s rainbow of colours. These gaps, called absorption lines, allo ...
... The yellow-white light of the Sun (familiar to all) arises because the Sun is very hot and emits light at all visible wavelengths. However, in 1802, studies with prisms (similar to what was used in the laboratory) showed gaps in the Sun’s rainbow of colours. These gaps, called absorption lines, allo ...
Chapter
... Reynolds Stress Model (RSM)), in which a transport equation of the Reynolds tensor (turbulent strain tensor) has been used. This model appears to be suitable to take into account intermediate turbulence areas where recirculation phenomena and streamlines of high curvature occur [1]. 2.2. Dispersed p ...
... Reynolds Stress Model (RSM)), in which a transport equation of the Reynolds tensor (turbulent strain tensor) has been used. This model appears to be suitable to take into account intermediate turbulence areas where recirculation phenomena and streamlines of high curvature occur [1]. 2.2. Dispersed p ...
Notations for today’s lecture (1 ) A complete set of ;
... Φα({x} ; t ) = < 0 | eitH/ħ Ψ(x1)Ψ(x2)...Ψ(xN)e−itH/ħ | α > (Trick question: Is this the Schroedinger picture or the Heisenberg picture?) Note that Φα is not an expectation value. The N factors of Ψ annihilate the particles, ...
... Φα({x} ; t ) = < 0 | eitH/ħ Ψ(x1)Ψ(x2)...Ψ(xN)e−itH/ħ | α > (Trick question: Is this the Schroedinger picture or the Heisenberg picture?) Note that Φα is not an expectation value. The N factors of Ψ annihilate the particles, ...
Exam - UF Physics
... (2) Blacken the circle of your intended answer completely, using a number 2 pencil. Do not make any stray marks or the answer sheet may not read properly. (3) The answers are rounded off. Choose the closest to exact. There is no penalty for guessing. >>>>>>>>WHEN YOU FINISH <<<<<<<< Hand in the gree ...
... (2) Blacken the circle of your intended answer completely, using a number 2 pencil. Do not make any stray marks or the answer sheet may not read properly. (3) The answers are rounded off. Choose the closest to exact. There is no penalty for guessing. >>>>>>>>WHEN YOU FINISH <<<<<<<< Hand in the gree ...
January - Life Learning Cloud
... A stone is thrown vertically upwards with speed 16 m s–1 from a point h metres above the ground. The stone hits the ground 4 s later. Find (a) the value of h, ...
... A stone is thrown vertically upwards with speed 16 m s–1 from a point h metres above the ground. The stone hits the ground 4 s later. Find (a) the value of h, ...
Document
... Like photons and electrons, protons, neutrons, atoms, and even molecules have wave properties ...
... Like photons and electrons, protons, neutrons, atoms, and even molecules have wave properties ...
Atomic Structure 1. Historical perspective of the model of the atom a
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
Lec-22_Strachan
... Electrons collected at C and passing through the ammeter create a current in the circuit C is maintained at a positive potential by the power supply No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated ...
... Electrons collected at C and passing through the ammeter create a current in the circuit C is maintained at a positive potential by the power supply No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated ...
Matter
... properties from the elements of which they are composed. Chemical bonds are the forces that hold the elements together in a compound creating a state of stability. ...
... properties from the elements of which they are composed. Chemical bonds are the forces that hold the elements together in a compound creating a state of stability. ...
Quantum Mechanics: PHL555 Tutorial 2
... Is an eigenfunction of L2 ? If so what is its corresponding eigenvalue. If not what are the possible values we shall obtain when we shall measure L2 . (b) What are the probabilities of finding out the particle in various m states? 4. (a) A particle is in a spherically symmetric potential is known ...
... Is an eigenfunction of L2 ? If so what is its corresponding eigenvalue. If not what are the possible values we shall obtain when we shall measure L2 . (b) What are the probabilities of finding out the particle in various m states? 4. (a) A particle is in a spherically symmetric potential is known ...
The Physics of Particle Detectors
... At the Cavendish Lab Thompson and Rutherford found that irradiating a gas with X-rays increased it’s conductivity suggesting that X-rays produced ions in the gas. Wilson used an X-Ray tube to irradiate his Chamber and found ‘a very great increase in the number of the drops’, confirming the hypothesi ...
... At the Cavendish Lab Thompson and Rutherford found that irradiating a gas with X-rays increased it’s conductivity suggesting that X-rays produced ions in the gas. Wilson used an X-Ray tube to irradiate his Chamber and found ‘a very great increase in the number of the drops’, confirming the hypothesi ...
Document
... Before the collision, two sheets of mutually transverse color electric and color magnetic fields. Boosted Coulomb fields Random in color ...
... Before the collision, two sheets of mutually transverse color electric and color magnetic fields. Boosted Coulomb fields Random in color ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.