unification of couplings
... emitted as radiation when the quarks or leptons accelerate, and even changing one kind of quark or lepton into another. The original and most familiar gauge theory is also the most basic. Quantum electrodynamics is properly understood, in modern terms, to be neither more nor less than the theory of ...
... emitted as radiation when the quarks or leptons accelerate, and even changing one kind of quark or lepton into another. The original and most familiar gauge theory is also the most basic. Quantum electrodynamics is properly understood, in modern terms, to be neither more nor less than the theory of ...
Chapter 4: The Structure of the Atom &
... o Proton – positive charge _________ 1.673x10-24 g o Neutron – neutral charge ________ About the same size as a proton: 1.675x10-24 g o Electron – negative charge _______ 1/1840 the size of a proton or neutron (9.11x10-28 g). However has equal (but opposite) charge of a proton. ...
... o Proton – positive charge _________ 1.673x10-24 g o Neutron – neutral charge ________ About the same size as a proton: 1.675x10-24 g o Electron – negative charge _______ 1/1840 the size of a proton or neutron (9.11x10-28 g). However has equal (but opposite) charge of a proton. ...
useful relations in quantum field theory
... to derive some of these relationships if the derivations are straightforward but many are just quoted. One of the most frustrating events for me is to find some formula and not know what conventions they are using. In this report I follow the Peskin and Schroeder conventions which I detail in the ne ...
... to derive some of these relationships if the derivations are straightforward but many are just quoted. One of the most frustrating events for me is to find some formula and not know what conventions they are using. In this report I follow the Peskin and Schroeder conventions which I detail in the ne ...
MiniQuiz 3
... DeBroglie proposed that the electron had wave properties, as well as particle properties. He proposed that the wavelength of a particle was related to the mass through the equation λ = h/mυ, where υ is the velocity. His original proposal was based on: a•) b) c) d) e) ...
... DeBroglie proposed that the electron had wave properties, as well as particle properties. He proposed that the wavelength of a particle was related to the mass through the equation λ = h/mυ, where υ is the velocity. His original proposal was based on: a•) b) c) d) e) ...
Cathode Ray Tubes and The JJ Thompson Experiment
... Famed physicist J.J. Thompson took the cathode ray a step further. First he set up a cathode ray tube that deflected the electron ray using a second set of electrically charged plates (aka yoke), similar to the ...
... Famed physicist J.J. Thompson took the cathode ray a step further. First he set up a cathode ray tube that deflected the electron ray using a second set of electrically charged plates (aka yoke), similar to the ...
AS_Unit1_Particle_10_Conservation_Rules
... (P) Baryon 0 ----> 0 + 0 (Baryon number is conserved) (P) Therefore it can proceed ...
... (P) Baryon 0 ----> 0 + 0 (Baryon number is conserved) (P) Therefore it can proceed ...
Module 11 - FacStaff Home Page for CBU
... What are some of the things that we should notice about these expressions for these physical quantities? First of all, remember that all of them revert to the classical, Newtonian expressions if u << c. We can get by just fine with saying that mass doesn’t change with speed and that kinetic energy ...
... What are some of the things that we should notice about these expressions for these physical quantities? First of all, remember that all of them revert to the classical, Newtonian expressions if u << c. We can get by just fine with saying that mass doesn’t change with speed and that kinetic energy ...
8th Grade: First Semester Final Review
... 1. Sample answer: The individual components of a heterogeneous mixture can be seen; the individual components of a homogeneous mixture cannot be seen. The individual components of a homogeneous mixture are evenly mixed; the individual components of a heterogeneous mixture are not evenly mixed. 2. Sa ...
... 1. Sample answer: The individual components of a heterogeneous mixture can be seen; the individual components of a homogeneous mixture cannot be seen. The individual components of a homogeneous mixture are evenly mixed; the individual components of a heterogeneous mixture are not evenly mixed. 2. Sa ...
Unit 2 Review KEY
... Photoelectric Effect – an emission of electrons from a metal when light shines on a metal. Quantum – minimum quantity of energy that can be lost or gained by an atom. Photon – particle of electromagnetic radiation having zero mass and carrying a quantum of energy. Heisenberg uncertainty principle – ...
... Photoelectric Effect – an emission of electrons from a metal when light shines on a metal. Quantum – minimum quantity of energy that can be lost or gained by an atom. Photon – particle of electromagnetic radiation having zero mass and carrying a quantum of energy. Heisenberg uncertainty principle – ...
May 2001
... particles. This can happen when there is radio emission from a pulsar, and these signals propagate through clouds of charged particles in deep space before being detected on Earth. A linearly polarized radio wave will induce a charged current in the cloud which is proportional to the timedependent e ...
... particles. This can happen when there is radio emission from a pulsar, and these signals propagate through clouds of charged particles in deep space before being detected on Earth. A linearly polarized radio wave will induce a charged current in the cloud which is proportional to the timedependent e ...
here
... • We are edging into the 46th, exploring the TeV energy scale. • The Standard Model of elementary particles is 50 years old. • It is alive and kicking. • The last missing piece of the SM been found. • The SM is not a complete theory! • We hope to get hints of what lies beyond in the next LHC run. ...
... • We are edging into the 46th, exploring the TeV energy scale. • The Standard Model of elementary particles is 50 years old. • It is alive and kicking. • The last missing piece of the SM been found. • The SM is not a complete theory! • We hope to get hints of what lies beyond in the next LHC run. ...
The Wave
... of light particles, but its NOT increasing the energy of each one! However, if the energy of these “light particle” is related to their frequency, this would explain why higher frequency light can knock the electrons out of their atoms, but low frequency light ...
... of light particles, but its NOT increasing the energy of each one! However, if the energy of these “light particle” is related to their frequency, this would explain why higher frequency light can knock the electrons out of their atoms, but low frequency light ...
Exit Slip: Atomic Structure and Nuclear Chemistry-1
... can be seen that the combined value of mass plus energy is conserved in these reactions. Which of these is the BEST example of such a reaction (11.b)? A. conversion of prehistoric plants to fossil fuels B. sudden explosive reactions with gaseous products C. splitting of a uranium nucleus into barium ...
... can be seen that the combined value of mass plus energy is conserved in these reactions. Which of these is the BEST example of such a reaction (11.b)? A. conversion of prehistoric plants to fossil fuels B. sudden explosive reactions with gaseous products C. splitting of a uranium nucleus into barium ...
Study Guide Matter: Building Blocks of the Universe
... Test consists of Multiple Choice, filling in charts with missing data and short answer. You should be prepared to answer questions on these topics. * Know the key people in the history of the atom and their contribution to our understanding of the atom. These should be in your lab book conclusion fo ...
... Test consists of Multiple Choice, filling in charts with missing data and short answer. You should be prepared to answer questions on these topics. * Know the key people in the history of the atom and their contribution to our understanding of the atom. These should be in your lab book conclusion fo ...
Chapter 5 Worksheet 1
... 1. State Heisenberg’s Uncertainty Principle as it pertains to atomic theory. It is impossible to determine the exact location and momentum of a moving object at the same time. 2. Why does the observation of a very small object such as an electron cause the electron to have its motion changed? The po ...
... 1. State Heisenberg’s Uncertainty Principle as it pertains to atomic theory. It is impossible to determine the exact location and momentum of a moving object at the same time. 2. Why does the observation of a very small object such as an electron cause the electron to have its motion changed? The po ...
Quantum Mechanics
... where a is the Bohr radius. Apply now an electric field E degenerate perturbation theory to first order. a. What is the perturbation Hamiltonian H 0 ? b. What is the matrix (secular) equation for H 0 ? Show that there are only two non-zero matrix elements. c. What are the eigenvectors, eigenvalues a ...
... where a is the Bohr radius. Apply now an electric field E degenerate perturbation theory to first order. a. What is the perturbation Hamiltonian H 0 ? b. What is the matrix (secular) equation for H 0 ? Show that there are only two non-zero matrix elements. c. What are the eigenvectors, eigenvalues a ...
Elementary particle
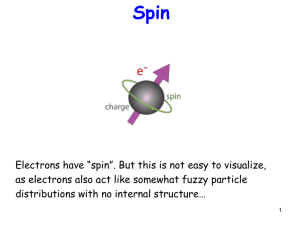
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.