Word Format
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
Problem Set 9: Groups & Representations Graduate Quantum I Physics 6572 James Sethna
... means that the p-electron orbitals do indeed stay an irreducible representation when the spherical symmetry is broken down to cubic, and hence will stay degenerate.) Check that our character is indeed orthogonal to the other rows in the table. Check that the ‘dot product’ of our character with the i ...
... means that the p-electron orbitals do indeed stay an irreducible representation when the spherical symmetry is broken down to cubic, and hence will stay degenerate.) Check that our character is indeed orthogonal to the other rows in the table. Check that the ‘dot product’ of our character with the i ...
Read Notes #1
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
Mn6 1 Many-particle Systems, 6 Fermion gas at low temperature At
... Example: What is PF for the conduction electrons in gold? Solution: Again, using ρ = 58 nm–3, we find PF = 128 eV/nm3. The latter is not as directly informative as expressing PF in macroscopic units—i.e., converting eV to J and nm to m. When this is done we find PF = 2x1010 N/m2. As 1 atm = 105 N/m2 ...
... Example: What is PF for the conduction electrons in gold? Solution: Again, using ρ = 58 nm–3, we find PF = 128 eV/nm3. The latter is not as directly informative as expressing PF in macroscopic units—i.e., converting eV to J and nm to m. When this is done we find PF = 2x1010 N/m2. As 1 atm = 105 N/m2 ...
PART1 - FacStaff Home Page for CBU
... 1. At a particular instant a particle of mass m A = 5 mg and charge of qA = 5 Coul is located at the origin. A second particle of mass mb = 2 mg and charge of qB = -6 Coul is located at that same instant at a position (-5 m, +3 m) relative to the origin. a) What is the force on qA due to the prese ...
... 1. At a particular instant a particle of mass m A = 5 mg and charge of qA = 5 Coul is located at the origin. A second particle of mass mb = 2 mg and charge of qB = -6 Coul is located at that same instant at a position (-5 m, +3 m) relative to the origin. a) What is the force on qA due to the prese ...
first chapter - damtp - University of Cambridge
... restriction, and classical mechanics is perfectly adequate to describe the system. The e ects of quantum mechanics are generally only important for submicroscopic systems. The chemistry of an atom is determined by the charge on its nucleus. Thus atoms whose nuclei di er only in the number of neutron ...
... restriction, and classical mechanics is perfectly adequate to describe the system. The e ects of quantum mechanics are generally only important for submicroscopic systems. The chemistry of an atom is determined by the charge on its nucleus. Thus atoms whose nuclei di er only in the number of neutron ...
1 ψ ω ω ω ψ ψ ψ
... for 0 ≤ x ≤ L and zero otherwise. (a) Determine the expectation value of x. (b) Determine the probability of finding the particle near L/2, by calculating the probability that the particle lies in the range 0.490L ≤ x ≤ 0.510L. (c) What If? Determine the probability of finding the particle near L/4, ...
... for 0 ≤ x ≤ L and zero otherwise. (a) Determine the expectation value of x. (b) Determine the probability of finding the particle near L/2, by calculating the probability that the particle lies in the range 0.490L ≤ x ≤ 0.510L. (c) What If? Determine the probability of finding the particle near L/4, ...
stationary state
... • When an electron is in one of the quantized orbits, it does not emit any electromagnetic radiation; thus, the electron is said to be in a stationary state. • The electron can make a discontinuous emission, or quantum jump, from one stationary state to another. During this transition it does emit r ...
... • When an electron is in one of the quantized orbits, it does not emit any electromagnetic radiation; thus, the electron is said to be in a stationary state. • The electron can make a discontinuous emission, or quantum jump, from one stationary state to another. During this transition it does emit r ...
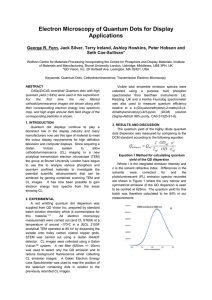
Fulltext
... In Figure 2a the high angle annular dark field (HAADF) image shows the HAADF STEM image of the QD ensemble and figure 2b shows for the first time by us the red light filtered CL image. There is a clear distribution of sizes present, see regions shown inside the triangles. The larger particles show C ...
... In Figure 2a the high angle annular dark field (HAADF) image shows the HAADF STEM image of the QD ensemble and figure 2b shows for the first time by us the red light filtered CL image. There is a clear distribution of sizes present, see regions shown inside the triangles. The larger particles show C ...
heavyions - Indico
... What are heavy ions about? A brief history of heavy-ion collisions What happens in a heavy ion collision? What are the essential observables? What are the theoretical approaches? What have we learnt from previous experiments? What do we expect from heavy ions at LHC? What do we expect from fixed-tar ...
... What are heavy ions about? A brief history of heavy-ion collisions What happens in a heavy ion collision? What are the essential observables? What are the theoretical approaches? What have we learnt from previous experiments? What do we expect from heavy ions at LHC? What do we expect from fixed-tar ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood
... 3. ______ _____________ unit of an ___________ and maintain the ______________ of that element 4. ____________ ___________ unit of a ___________; maintaining _____________ of the compound 5. ______________ matter that is composed of one kind of ________ (e.g. sulfur [__]; carbon [__]) a. each ...
... 3. ______ _____________ unit of an ___________ and maintain the ______________ of that element 4. ____________ ___________ unit of a ___________; maintaining _____________ of the compound 5. ______________ matter that is composed of one kind of ________ (e.g. sulfur [__]; carbon [__]) a. each ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.