A = 27
... #32) Al+3 has lost 3 electrons (each + charge represents a lost electron). The neutral atom has 13 protons, thus there are 13 electrons in the neutral atom. If three e- were lost 10, are remaining. ANS-4 #33 The excited state must have the same # of electrons as the neutral atom, however one or more ...
... #32) Al+3 has lost 3 electrons (each + charge represents a lost electron). The neutral atom has 13 protons, thus there are 13 electrons in the neutral atom. If three e- were lost 10, are remaining. ANS-4 #33 The excited state must have the same # of electrons as the neutral atom, however one or more ...
Notes for Momentum
... How To Make Physics Pay We will now look at a way of calculating where the pool balls will go. To do this we will also have to use the Law of Conservation of Energy ...
... How To Make Physics Pay We will now look at a way of calculating where the pool balls will go. To do this we will also have to use the Law of Conservation of Energy ...
4. One mole of a monatomic ideal gas initially at temperature 0 T
... density. Assuming the electrons are degenerate and non‐relativistic find the average energy of the electrons in terms of M, R and fundamental constants. b. (10) Repeat part a) assuming the electrons are relativistic. c. (10) Assume the radius shrinks by a factor of 2 so R′ = R / 2 while the mass rem ...
... density. Assuming the electrons are degenerate and non‐relativistic find the average energy of the electrons in terms of M, R and fundamental constants. b. (10) Repeat part a) assuming the electrons are relativistic. c. (10) Assume the radius shrinks by a factor of 2 so R′ = R / 2 while the mass rem ...
Electric charges, Coulomb`s law, and Electric Field
... Ebonite rod & Fur Negatively charged ebonite rod Glass rod & Silk ...
... Ebonite rod & Fur Negatively charged ebonite rod Glass rod & Silk ...
proposition de stage - Laboratoire de l`Accélérateur Linéaire
... The current target of particle physics is a discovery of a signal beyond the Standard Model (SM). Various models beyond SM have been proposed based on strong theoretical motivations. Having the Large Hadron Collider (CERN/Geneva) providing vast number of experimental results, it is now that some of ...
... The current target of particle physics is a discovery of a signal beyond the Standard Model (SM). Various models beyond SM have been proposed based on strong theoretical motivations. Having the Large Hadron Collider (CERN/Geneva) providing vast number of experimental results, it is now that some of ...
English (MS Word) - CMS DocDB Server
... The excess is seen in a range of final states involving photons, electrons, muons and hadrons. ...
... The excess is seen in a range of final states involving photons, electrons, muons and hadrons. ...
Fig. 1: Four charged rods.
... 1. Two parallel plates having charges of equal magnitude but opposite sign are separated by 12.0 cm. Each plate has a surface charge density of 36.0 nC/m2 . A proton is released from rest at the positive plate. Determine (a) the potential difference between the plates. (b) the kinetic energy of the p ...
... 1. Two parallel plates having charges of equal magnitude but opposite sign are separated by 12.0 cm. Each plate has a surface charge density of 36.0 nC/m2 . A proton is released from rest at the positive plate. Determine (a) the potential difference between the plates. (b) the kinetic energy of the p ...
Relativity Problem Set 9
... (b) Recall that for a beam of free particles, ψ ∗ (x)ψ(x) gives the number of particles per unit distance. Using this, discuss whether it would be possible to find a particle in the region x > 0 if a measurement were made on the system. (c) What is the probability that an incident particle will be r ...
... (b) Recall that for a beam of free particles, ψ ∗ (x)ψ(x) gives the number of particles per unit distance. Using this, discuss whether it would be possible to find a particle in the region x > 0 if a measurement were made on the system. (c) What is the probability that an incident particle will be r ...
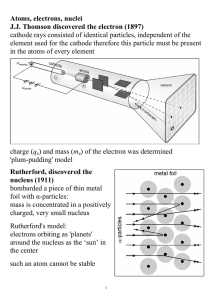
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercur ...
... energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercur ...
Mass spectrometer, Hall effect, force on wire
... • composed of two hollow copper dees that are immersed in a uniform magnetic field & connected to an oscillating voltage source. ...
... • composed of two hollow copper dees that are immersed in a uniform magnetic field & connected to an oscillating voltage source. ...
Group-Symmetries and Quarks - USC Department of Physics
... M=mA+mB. The C’s are calculated by using Symbolically, ...
... M=mA+mB. The C’s are calculated by using Symbolically, ...
Pair production processes and flavor in gauge
... [1–4]. In the electroweak sector, this leads to an apparent contradiction. Strictly speaking, the elementary particles, i.e., the fields of the Lagrangian, the Higgs, the gauge bosons, but also the fermions, are not gaugeinvariant states [1–3]. However, treating them like they would be in perturbati ...
... [1–4]. In the electroweak sector, this leads to an apparent contradiction. Strictly speaking, the elementary particles, i.e., the fields of the Lagrangian, the Higgs, the gauge bosons, but also the fermions, are not gaugeinvariant states [1–3]. However, treating them like they would be in perturbati ...
Unit 3 - Chemistry
... • Expand without limit to fill any space. • _______________ - describes the gaseous state of a substance that is generally a liquid or solid at room temperature (different than a gas). ...
... • Expand without limit to fill any space. • _______________ - describes the gaseous state of a substance that is generally a liquid or solid at room temperature (different than a gas). ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.