
Chemistry Review - Net Start Class
... • An atom is the smallest unit of an element that upholds all of the properties of that element. ...
... • An atom is the smallest unit of an element that upholds all of the properties of that element. ...
Structure of Matter
... Neutrons don’t have electrostatic repulsion - their energy levels are slightly lower than proton energy levels. A lower overall energy state can therefore be reached by turning some protons into extra neutrons. At some point even the additional neutrons cannot protect the nucleus from the electrosta ...
... Neutrons don’t have electrostatic repulsion - their energy levels are slightly lower than proton energy levels. A lower overall energy state can therefore be reached by turning some protons into extra neutrons. At some point even the additional neutrons cannot protect the nucleus from the electrosta ...
Atoms, Ions and Molecules The Building Blocks of Matter
... because most of the -particles passed through undeflected • The nucleus is very dense and positively charged because some of the -particles were repulsed and deflected • Electrons occupy the space around the nucleus • The atom is electrically neutral ...
... because most of the -particles passed through undeflected • The nucleus is very dense and positively charged because some of the -particles were repulsed and deflected • Electrons occupy the space around the nucleus • The atom is electrically neutral ...
Accelerate This! - University of Houston
... to make tiny things go really fast Daniel Friedman, St. John’s School ...
... to make tiny things go really fast Daniel Friedman, St. John’s School ...
Physics 3204
... energy of 18 J. What would be the electric potential energy at the same point for a charge of 6.0 μC? 6. At a point 12 cm from a point charge the electric potential is 12.0 V. What will be the potential 6.0 cm away? 7. A proton is moved through a potential difference of 110 V. How much work is done? ...
... energy of 18 J. What would be the electric potential energy at the same point for a charge of 6.0 μC? 6. At a point 12 cm from a point charge the electric potential is 12.0 V. What will be the potential 6.0 cm away? 7. A proton is moved through a potential difference of 110 V. How much work is done? ...
MS PowerPoint template
... We report the manipulation of glass micro spheres having a diameter of 3-10 μm using optical tweezers and with haptic feedback. We detect the position of a micro sphere manipulated in a fluid bed using a CCD camera and calculate the forces acting on it due to the optical trap and viscous drag. We es ...
... We report the manipulation of glass micro spheres having a diameter of 3-10 μm using optical tweezers and with haptic feedback. We detect the position of a micro sphere manipulated in a fluid bed using a CCD camera and calculate the forces acting on it due to the optical trap and viscous drag. We es ...
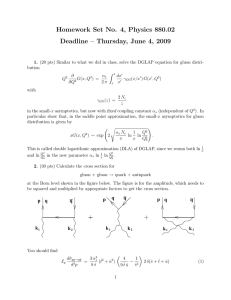
Homework Set No. 4, Physics 880.02
... in the small-x asymptotics, but now with fixed coupling constant αs (independent of Q2 ). In particular show that, in the saddle point approximation, the small-x asymptotics for gluon distribution is given by s ...
... in the small-x asymptotics, but now with fixed coupling constant αs (independent of Q2 ). In particular show that, in the saddle point approximation, the small-x asymptotics for gluon distribution is given by s ...
A protein molecule in an electrophoresis gel has a negative charge
... First, we are told to assume that each protein molecule has 30 excess electrons. We can find the net charge on a protein molecule by multiplying the number of excess electrons per molecule by the charge of each electron. We are told in part one of the electricity and magnetism reading that each elec ...
... First, we are told to assume that each protein molecule has 30 excess electrons. We can find the net charge on a protein molecule by multiplying the number of excess electrons per molecule by the charge of each electron. We are told in part one of the electricity and magnetism reading that each elec ...
How many significant figures are there in each of these
... SIGNIFICANT, still need to be included, so we know how big the number is! ...
... SIGNIFICANT, still need to be included, so we know how big the number is! ...
Anecdotes in the Lives of Some Prominent Physicists behind The
... to know what kind of thinking stimulated the amazing breakthroughs of these physicists. What motivated these scientists, what was the social climate like at that time, did they have any hobbies or interests that may have influenced their thoughts? For example, Louis de Broglie had a strong musical b ...
... to know what kind of thinking stimulated the amazing breakthroughs of these physicists. What motivated these scientists, what was the social climate like at that time, did they have any hobbies or interests that may have influenced their thoughts? For example, Louis de Broglie had a strong musical b ...
Chapter 3
... 1. History of the Atomic Model: a. Explain how the following scientists contributed to the development of the modern atomic theory. Describe their model and explain limitations. o Dalton’s Billiard Ball Model o Thomson’s Raisin Bun Model o Rutherford’s Nuclear Model o Bohr’s Planetary Model b. Expla ...
... 1. History of the Atomic Model: a. Explain how the following scientists contributed to the development of the modern atomic theory. Describe their model and explain limitations. o Dalton’s Billiard Ball Model o Thomson’s Raisin Bun Model o Rutherford’s Nuclear Model o Bohr’s Planetary Model b. Expla ...
Particles and Waves
... Most alphas passed straight through without much deflection. B. Every so often an alpha was deflected through a large angle. For each result, state what conclusion(s) were made about the structure of the atom. ...
... Most alphas passed straight through without much deflection. B. Every so often an alpha was deflected through a large angle. For each result, state what conclusion(s) were made about the structure of the atom. ...
arXiv:1501.01596v1 [cond-mat.mtrl-sci] 3 Jan 2015
... [2], the detection of low-energy neutrinos, radon and neutron counting. In this context, the Spherical Proportional Counter (SPC) was proposed in [3] as a candidate fulfilling all the requirements: a single readout channel reading a big gas volume (and mass); a potentially low intrinsic background l ...
... [2], the detection of low-energy neutrinos, radon and neutron counting. In this context, the Spherical Proportional Counter (SPC) was proposed in [3] as a candidate fulfilling all the requirements: a single readout channel reading a big gas volume (and mass); a potentially low intrinsic background l ...
The magnetic force on a charged particle
... the positive x direction. A uniform magnetic field of magnitude B exists in the negative z direction. You want to balance the magnetic force with an electric field so that the particle will continue along a straight line. The electric field should be in the 1) positive x direction. 2) positive z dir ...
... the positive x direction. A uniform magnetic field of magnitude B exists in the negative z direction. You want to balance the magnetic force with an electric field so that the particle will continue along a straight line. The electric field should be in the 1) positive x direction. 2) positive z dir ...
How stable are extra dimensions? - Theoretical High
... extremum of scalar potential leads to De Sitter space-time. ...
... extremum of scalar potential leads to De Sitter space-time. ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.


![arXiv:1606.09570v1 [physics.gen-ph] 29 Jun 2016](http://s1.studyres.com/store/data/017089913_1-b139408de069e5e90b03e592a6592573-300x300.png)














![arXiv:1501.01596v1 [cond-mat.mtrl-sci] 3 Jan 2015](http://s1.studyres.com/store/data/008057215_1-2593210d98eafff454da21c3b7b4536e-300x300.png)




