- Catalyst
... Modern Reassessment of the Atomic Theory 1. All matter is composed of atoms. Although atoms are composed of smaller particles (electrons, protons, and neutrons), the atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of a ...
... Modern Reassessment of the Atomic Theory 1. All matter is composed of atoms. Although atoms are composed of smaller particles (electrons, protons, and neutrons), the atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of a ...
... I 10. (1 0) The decay between two excited states of the nucle~isof 4 ' ~ iemits gamma ray of 1.3117 MeV. Tht luppe, state has a lifetime of 1.4ps, the lower state 3.0 ps. A) What is the fractional uncertainty AEIE in tht energy of the gainma ray? B) What is the percentage spread in wavelength of the ...
CHAPTER 9: Statistical Physics
... Fermi-Dirac Statistics Bose-Einstein Statistics Ludwig Boltzmann, who spent much of his life studying statistical mechanics, died in 1906 by his own hand. Paul Ehrenfest, carrying on his work, died similarly in 1933. Now it is our turn to study statistical mechanics. Perhaps it will be wise to appro ...
... Fermi-Dirac Statistics Bose-Einstein Statistics Ludwig Boltzmann, who spent much of his life studying statistical mechanics, died in 1906 by his own hand. Paul Ehrenfest, carrying on his work, died similarly in 1933. Now it is our turn to study statistical mechanics. Perhaps it will be wise to appro ...
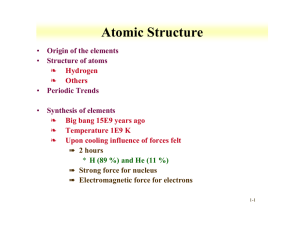
Atomic Structure Origin of the elements Structure of atoms Periodic Trends
... ❧ rapidly decay to form stable neutron rich nuclei • P process ❧ Proton capture, not as common ...
... ❧ rapidly decay to form stable neutron rich nuclei • P process ❧ Proton capture, not as common ...
-30- Section 9: f"
... 1. What would be the average intensity of an electromagnetic wave in which each cubic meter contained 5.00 J of energy? 2. When a CO molecule changes from the J = 4 to J = 3 rotational state, it emits a 1.91 x 10-3eV photon. What is this molecule's rotational kinetic energy in the J = 3 state? 3. In ...
... 1. What would be the average intensity of an electromagnetic wave in which each cubic meter contained 5.00 J of energy? 2. When a CO molecule changes from the J = 4 to J = 3 rotational state, it emits a 1.91 x 10-3eV photon. What is this molecule's rotational kinetic energy in the J = 3 state? 3. In ...
Electron Configuration and New Atomic Model
... challenged the wave theory of interaction between light and matter. • The photoelectric effect refers to the emission of electrons from a metal when light shines on it. • The wave theory of light predicted that any frequency of light would supply enough energy to eject an electron. • However, in ...
... challenged the wave theory of interaction between light and matter. • The photoelectric effect refers to the emission of electrons from a metal when light shines on it. • The wave theory of light predicted that any frequency of light would supply enough energy to eject an electron. • However, in ...
Electron Configuration - Warren County Public Schools
... challenged the wave theory of interaction between light and matter. • The photoelectric effect refers to the emission of electrons from a metal when light shines on it. • The wave theory of light predicted that any frequency of light would supply enough energy to eject an electron. • However, in thi ...
... challenged the wave theory of interaction between light and matter. • The photoelectric effect refers to the emission of electrons from a metal when light shines on it. • The wave theory of light predicted that any frequency of light would supply enough energy to eject an electron. • However, in thi ...
Karlsruhe School of Elementary Particle and Astroparticle Physics
... Neutrinos play an important role in cosmology and elementary particle physics. The most important open question is their mass, ...
... Neutrinos play an important role in cosmology and elementary particle physics. The most important open question is their mass, ...
Parity and Charge conjugation
... what we observe in nature. In order to prove that the CP is conserved in above two decays, we really need to check that the decay rates of both decays indentical or not by experiments. For the moment, let us assume that the CP is conserved in the weak interactions. We start with discussing the syste ...
... what we observe in nature. In order to prove that the CP is conserved in above two decays, we really need to check that the decay rates of both decays indentical or not by experiments. For the moment, let us assume that the CP is conserved in the weak interactions. We start with discussing the syste ...
Homework 4 Answer Key
... So, the error is about five one-hundredths of a percent. Thus, instead of an ionization potential of –13.6 eV, one would compute –13.6 eV (the error does not appear with only ! takes an excruciatingly sensitive instrument to measure the 3 significant digits). It difference (although the measurement ...
... So, the error is about five one-hundredths of a percent. Thus, instead of an ionization potential of –13.6 eV, one would compute –13.6 eV (the error does not appear with only ! takes an excruciatingly sensitive instrument to measure the 3 significant digits). It difference (although the measurement ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.